2025 Year-End Review: Pioneering the Era of Agentic AI in Life Sciences
Dec 23, 2025🚀 2025 was a defining year for life sciences—and a pivotal one for how AI is reshaping regulatory, medical, and clinical work.From industry stages around the world to real-world deployments, AlphaLife Sciences helped move the conversation from AI potential to AI impact. Agentic AI is no longer theoretical—it is actively transforming how teams author, reuse, and govern clinical and regulatory content at scale, with speed, precision, and confidence. 🧠⚙️In our 2025 Year-End Review, we reflect on the milestones, partnerships, and breakthroughs that marked a new era for intelligent, autonomous systems in life sciences—and how AuroraPrime RMA is helping organizations accelerate development while raising the bar for quality and compliance. 📈🧬If you work across regulatory, medical, or clinical domains and are thinking about what’s next for AI-driven transformation, this is a moment worth revisiting.
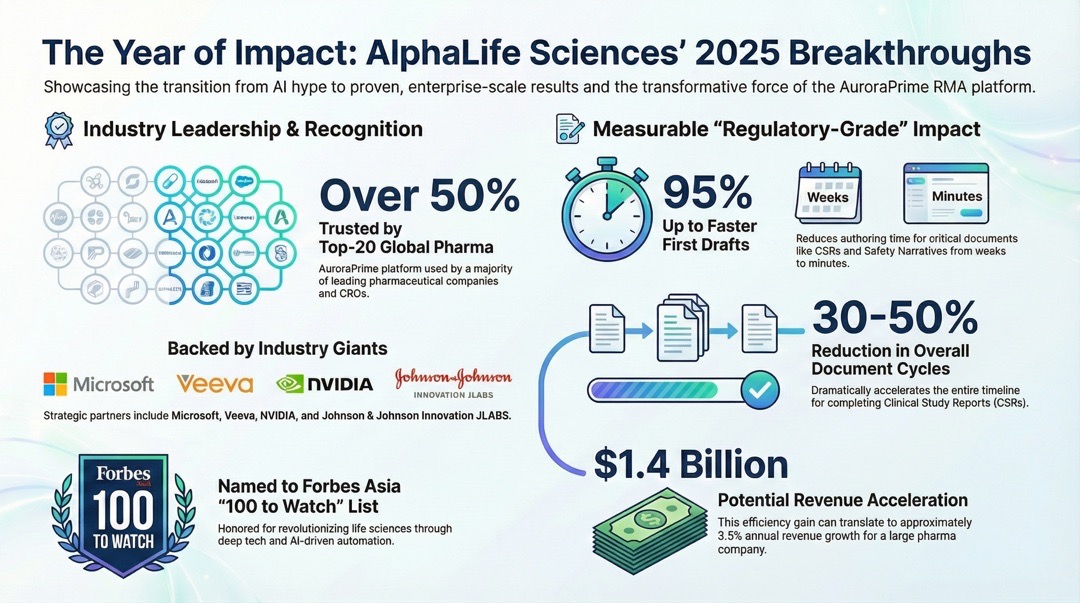
As we conclude a transformative year, AlphaLife Sciences stands at the forefront of a paradigm shift in clinical development. Throughout 2025, our mission remained clear: to accelerate drug development and make it more affordable by automating life-science content authoring through AuroraPrime RMA, our "regulatory-grade" AI platform. This year, we moved beyond the initial hype of Generative AI to deliver measurable, enterprise-scale impact for the world’s leading pharmaceutical organizations.
Global Industry Presence and Thought Leadership

In 2025, AlphaLife Sciences became a leading voice in the conversation regarding the future of medical writing and regulatory excellence. We participated in a wide array of premier industry events across the globe, reaching thousands of professionals in the regulatory, medical, and clinical sectors.
AMWA 2025 (Phoenix, AZ): As a Platinum Sponsor, we led the highly attended panel, “How Leading Global Pharma Embraces AI to Automate Regulatory and Medical Documents with Quality Control,” featuring leaders from Johnson & Johnson, ICON plc, and Microsoft.
DIA Global Annual Meeting (Washington D.C.): Our partner, Microsoft, showcased AuroraPrime RMA as the prime example of responsible AI in regulated environments, emphasizing its readiness for immediate, scalable deployment.
DIA Japan (Tokyo): We engaged with Asian stakeholders on the theme of "Tomorrow’s Normal," demonstrating how AI can cut document cycles by up to 90%.
DIA Europe: CEO Sharon Chen delivered real-world case studies showing how global teams are reducing first-draft authoring time from weeks to minutes.
BiotechX US (Philadelphia): Sharon Chen’s keynote addressed the integration of AI into core operations for "frontier firms," highlighting the shift toward high-quality automated clinical content.
RAPS Convergence & EMWA Virtual Conference: We joined regulatory and medical writing professionals to discuss maintaining non-negotiable standards of integrity while leveraging "Intelligent Agents" for end-to-end automation.
SCOPE US (Orlando): We demonstrated AuroraPrime’s ability to connect data sources across the clinical lifecycle, generating drafts in minutes with seamless Veeva RIM integration.
Significant Achievements and Industry Recognition
Our commitment to innovation was recognized by some of the most prestigious organizations in the world.
Forbes Asia 100 to Watch 2025: AlphaLife Sciences was honored on this list for our relentless drive to revolutionize life sciences through deep tech and AI-driven automation.
CDISC Gold Membership: We reached a strategic milestone by joining CDISC, allowing us to integrate global data standards directly into our platform for seamless, audit-ready compliance.
Strategic Partnerships: We expanded our ecosystem through the Veeva AI Partner Program and the availability of AuroraPrime Create on Microsoft AppSource. We also continue to be backed by the Microsoft for Startups Pegasus Program, NVIDIA Inception, and J&J Innovation JLABS.
Market Leadership: Today, AuroraPrime RMA is trusted by more than half of the Top-20 global pharmaceutical companies and leading CROs.
Measurable Industry Impact: Delivering "Regulatory-Grade" Results

In 2025, AuroraPrime RMA delivered tangible returns on investment by shifting medical writing from a manual, linear process to an AI-augmented workflow.
Accelerated Timelines: Our clients consistently reported a 30% to 50% reduction in overall document cycle times for Clinical Study Reports (CSRs).
Phenomenal Drafting Speed: We achieved up to a 90% reduction in first-draft time for CSRs and Protocols, and a 95% reduction for Safety Narratives.
Financial Advantage: This efficiency translates to roughly $1.4 billion in revenue acceleration for a large pharmaceutical company, or approximately 3.5% in annual revenue growth.
Uncompromising Quality: Our built-in AI Quality Control framework acts as a "second pair of eyes," detecting inconsistencies that humans might overlook and ensuring that every document meets the highest standards of regulatory integrity.
Community and Gratitude
Our success this year would not have been possible without the collaboration of our visionary partners and customers. We extend our deepest gratitude to Neil Garrett (Johnson & Johnson), Karen J. Devcich (ICON plc), Eric Henze and Eunice Youhanna (Microsoft), and Dr. John Jenkins and Dr. Ellis F. Unger for their invaluable perspectives and thought leadership. We thank the entire AlphaLife community for your trust as we work together to deliver life-saving treatments to patients faster.
A Forward-Looking Outlook: The Future is Orchestrated
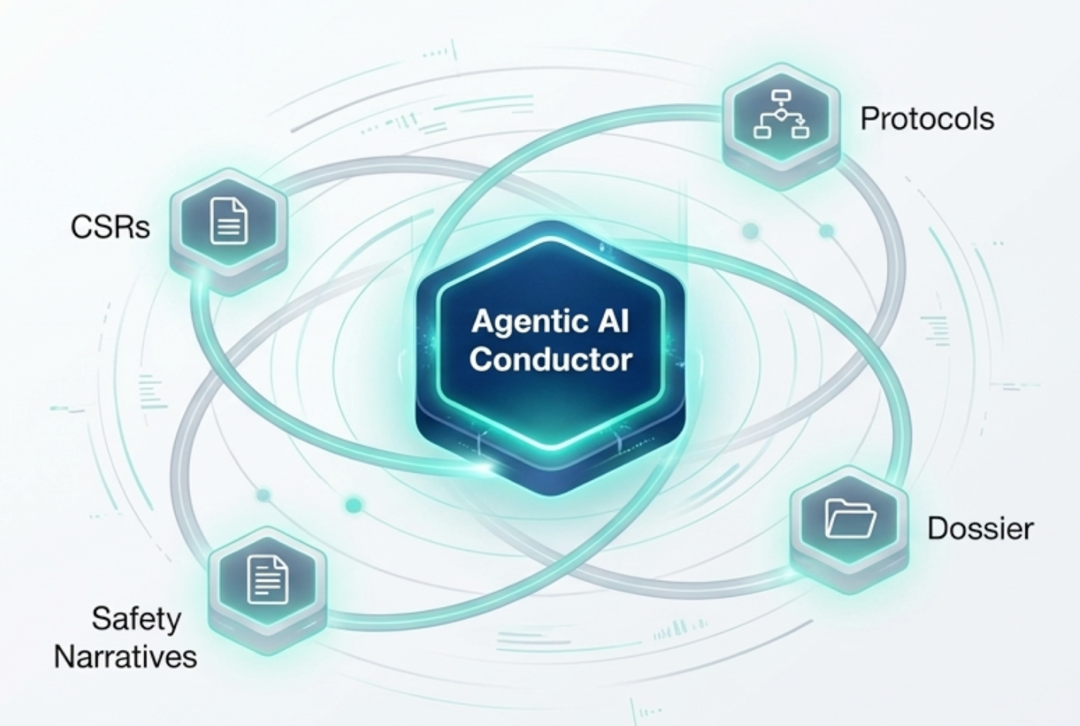
Looking ahead to 2026, AlphaLife Sciences is moving beyond AI-assisted drafting toward full workflow orchestration. Our focus will be on Agentic AI, where intelligent agents autonomously map the full hierarchy of information across submission packages to ensure unmatched consistency and quality.
We are also excited to scale our new CSR Self-Service program, designed to make high-end AI automation accessible for rapid use-cases. The future of regulatory excellence belongs to those who operationalize AI today, and we are committed to leading that transformation.
AlphaLife Sciences: Accelerating Innovation. Ensuring Integrity. Transforming the Future of Healthcare.





