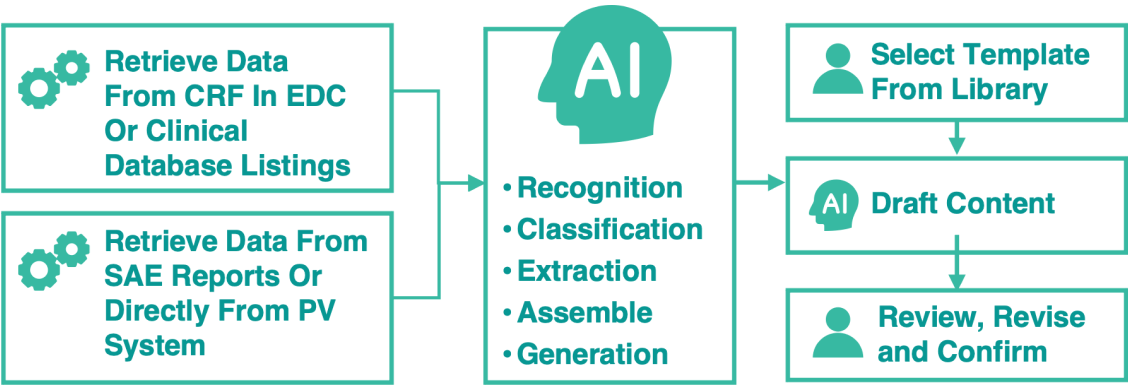
Flexible, Pre-built Templates
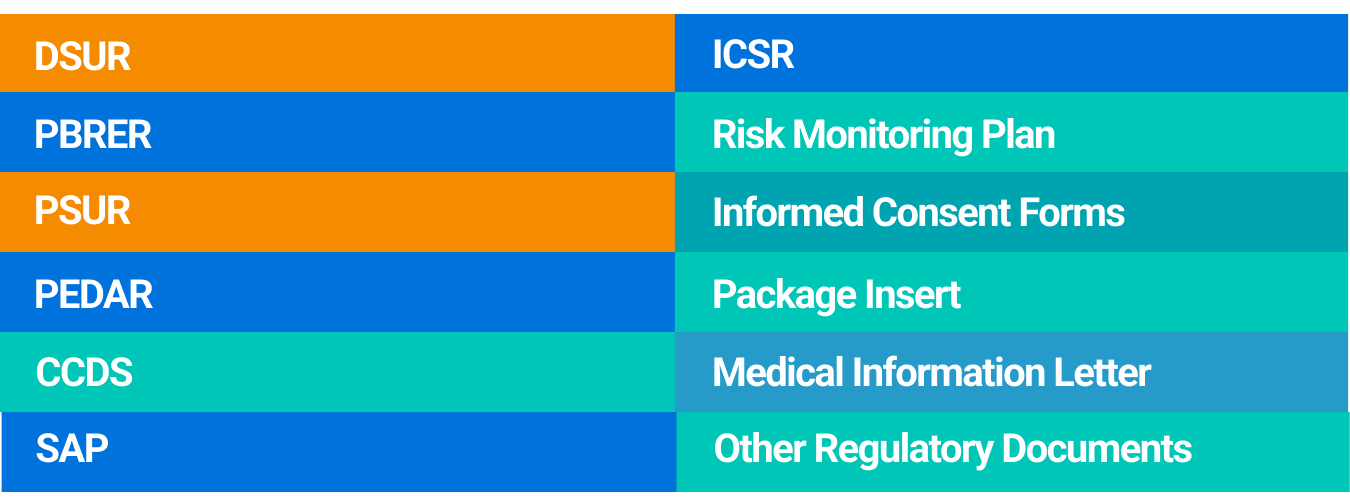
When using our AI writing tool, you can begin by browsing through AuroraPrime's library of pre-built templates designed for various regulatory documents. These templates provide a structured foundation, ensuring consistency and compliance from the outset for documents like DSURs and PSURs.
AI-Driven Drafting & Updates
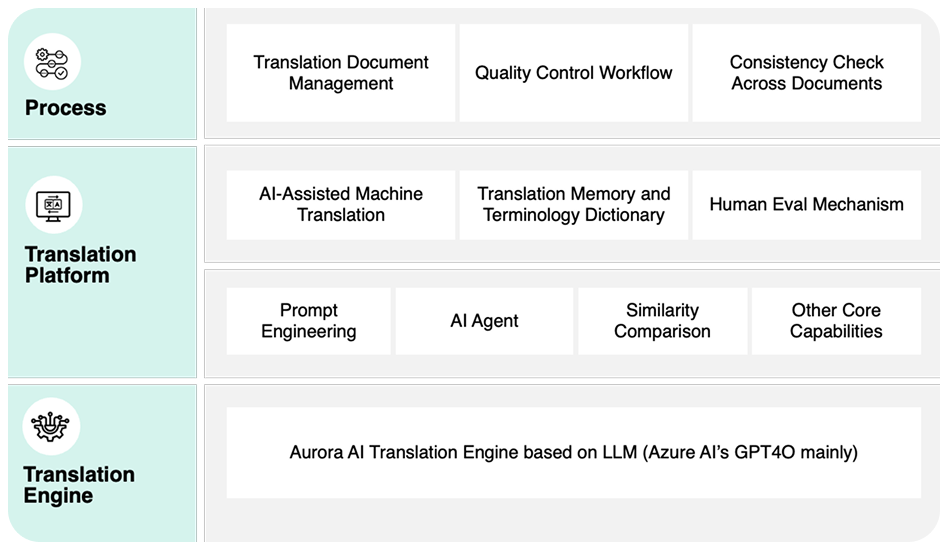
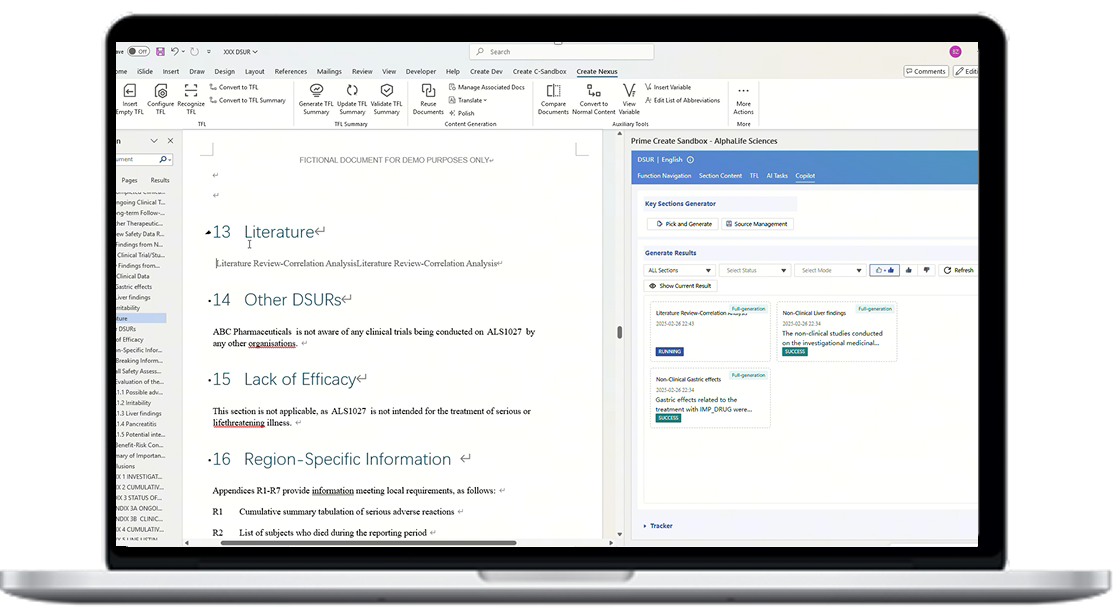
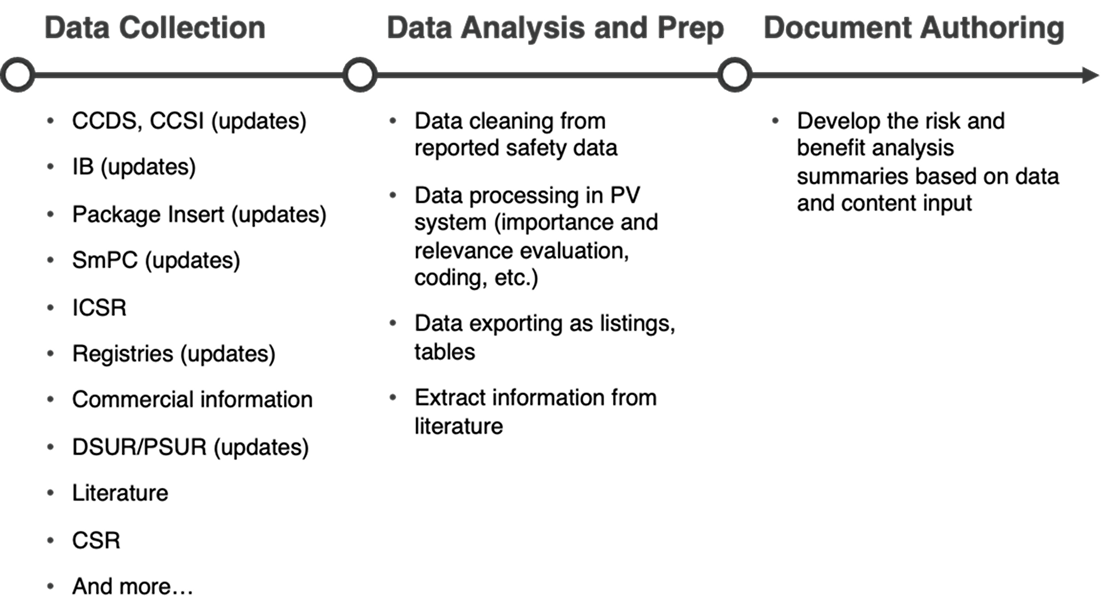
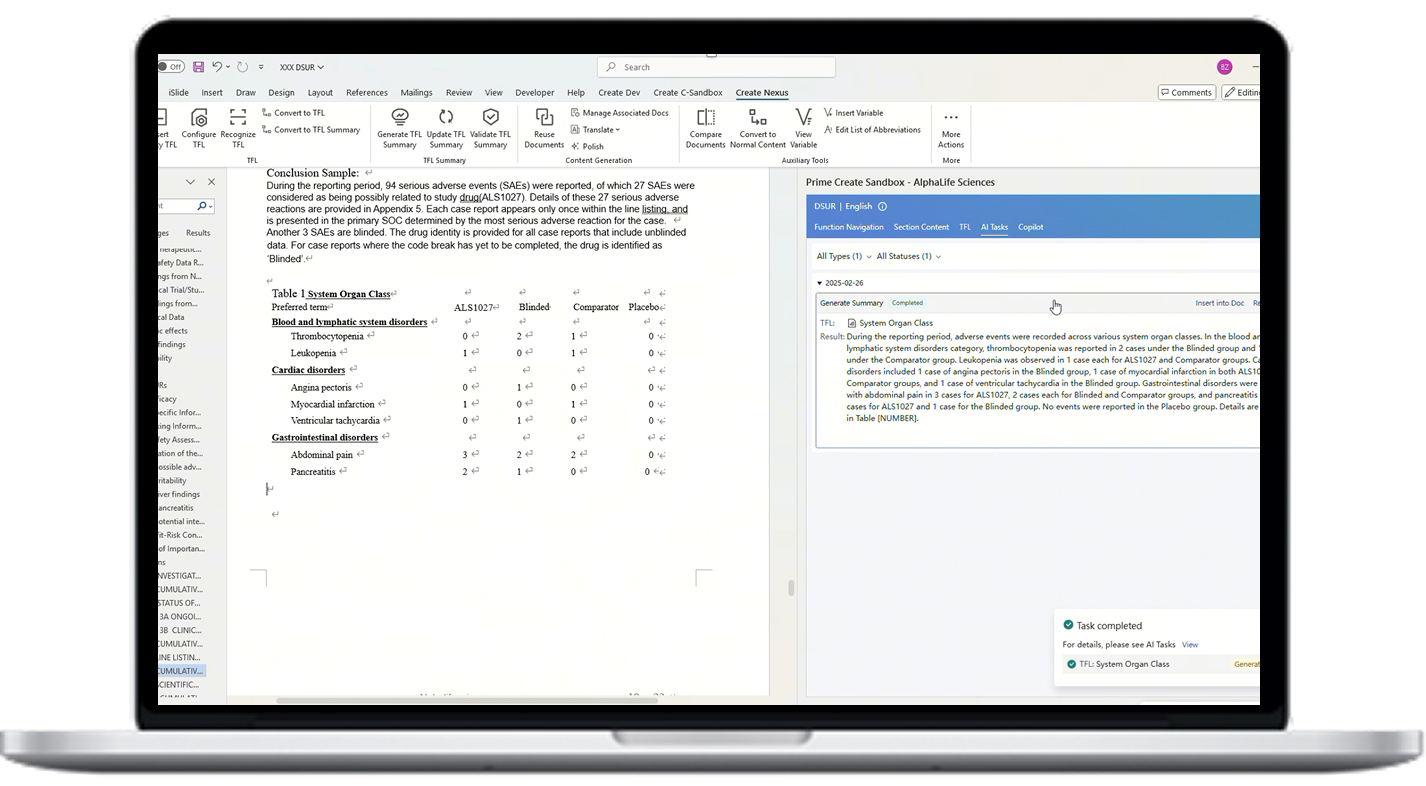
By leveraging AI and integration with Veeva RIM, AuroraPrime automates content creation by synthesizing upstream documents and data. This enables rapid generation of comprehensive drafts, significantly reducing manual effort.
Automated Update Triggers
With our regulatory document workflows, our AI software can facilitate seamless collaboration among teams. The platform enables real-time review, editing, and feedback, ensuring all stakeholders are aligned throughout the document lifecycle.
Collaborative Review & Version Control
Streamline the finalization and submission process of regulatory documentation with AuroraPrime's automated tools. The system ensures documents are complete, compliant, and ready for submission to regulatory authorities, reducing delays and errors.