7 Forms of Waste in Medical Writing and How to Eliminate Them
Nov 18, 2024The article explores seven types of inefficiencies in medical writing, adapted from lean manufacturing's "7 Wastes": overproduction, waiting, transport, overprocessing, inventory, motion, and defects. Each form of waste in medical writing, like excessive documentation or delays in feedback, disrupts workflow and reduces content quality. Using lean principles, enhanced by tools like AuroraPrime Create, can streamline processes, improve quality control, and boost productivity, leading to faster clinical documentation and better outcomes.

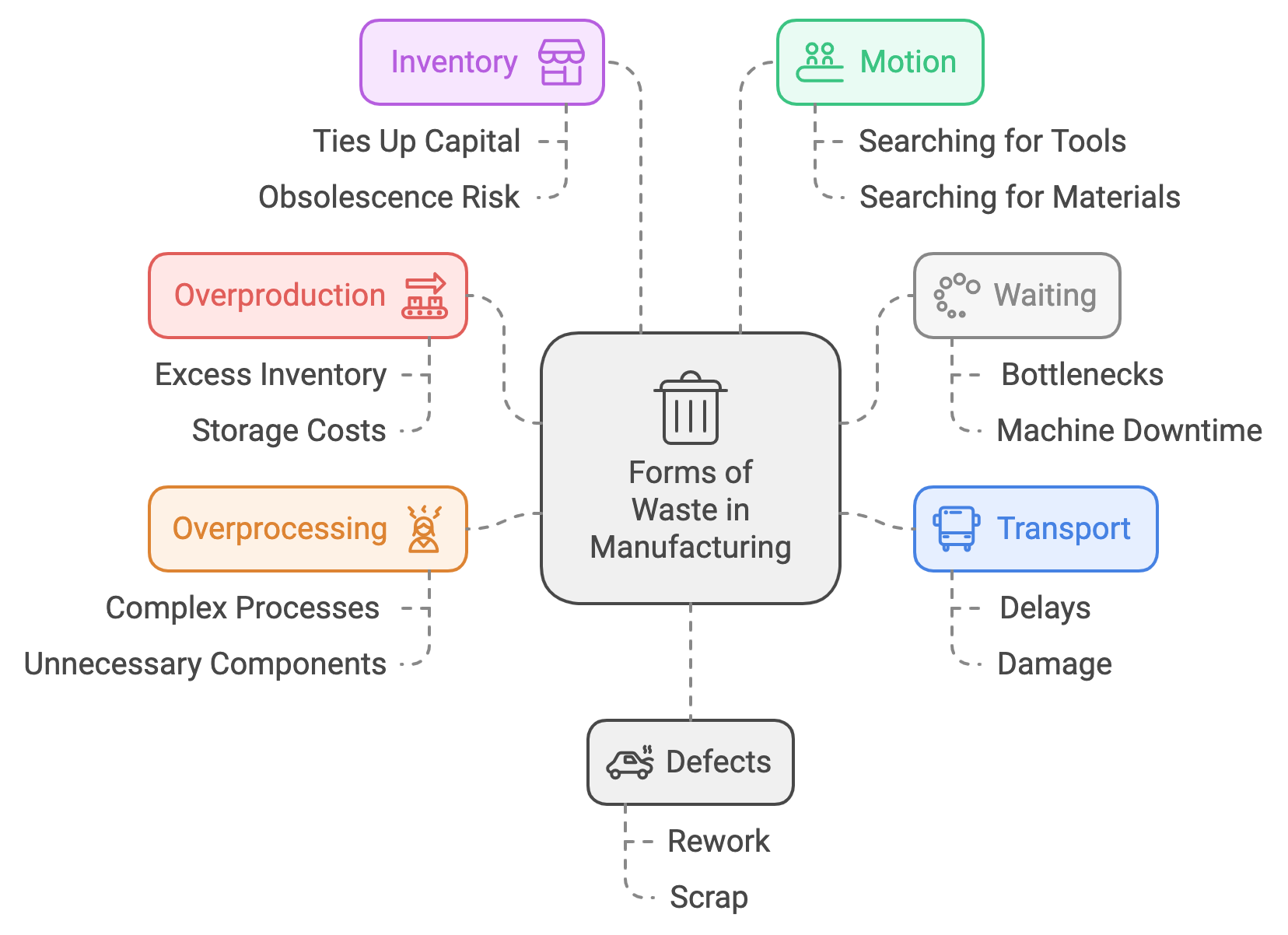
In manufacturing, seven recognized forms of waste—also known as the 7 Wastes or 7 Mudas—are core principles from Lean Manufacturing that originated within the Toyota Production System (TPS). These non-value-adding activities create inefficiencies throughout processes, and applying lean principles to eliminate them is essential for optimizing productivity.
These same lean principles are highly adaptable to medical writing, where inefficiencies can slow documentation workflows and compromise the quality of deliverables. By adopting a lean approach, medical writers can streamline document creation and improve overall quality, fostering faster and more effective communication within healthcare. Advanced tools like AuroraPrime Create from AlphaLife Sciences support these lean practices, enabling medical writers to enhance their workflow and reduce inefficiencies.
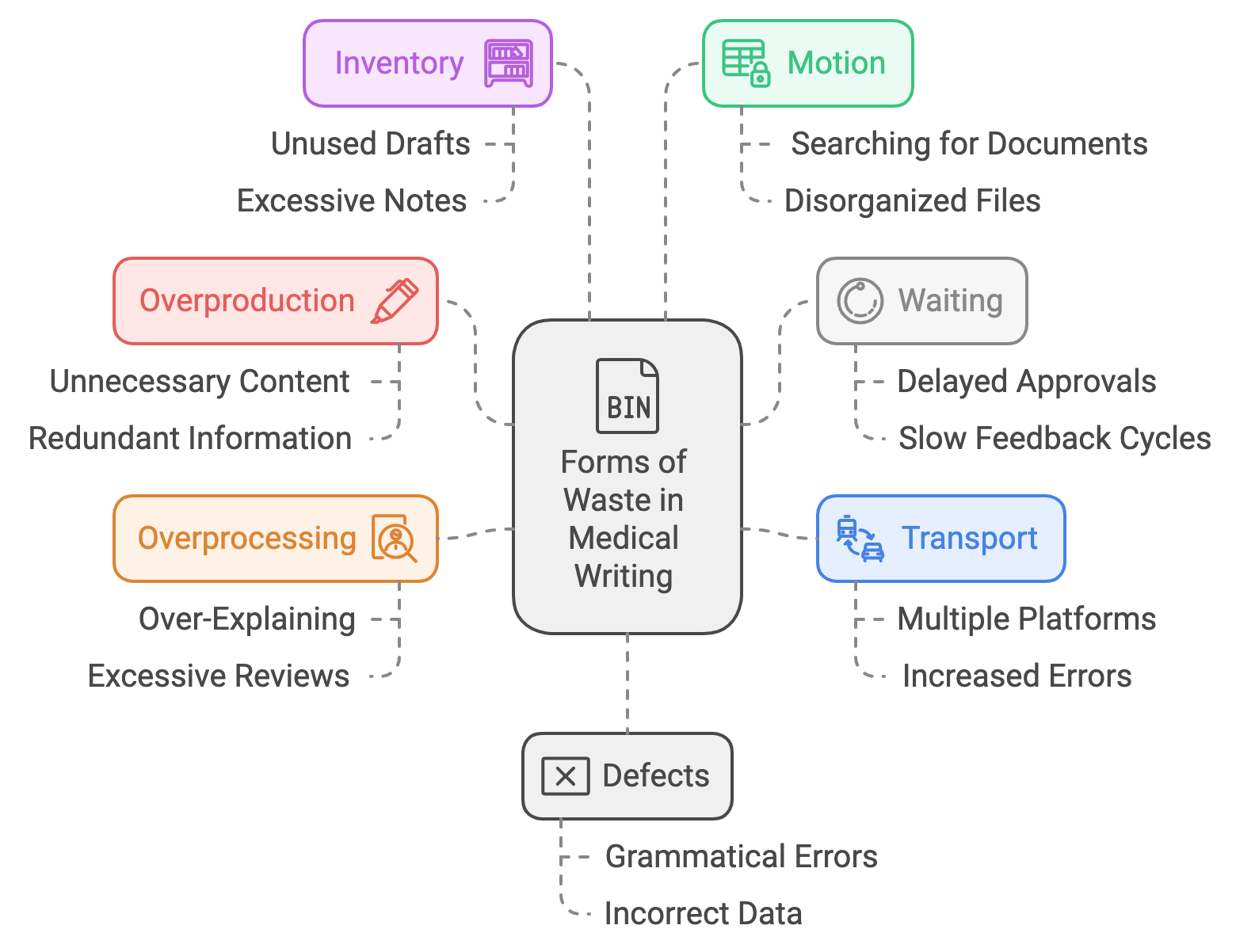
Overproduction
In manufacturing, overproduction occurs when more is produced than required or when production happens too early. This leads to excessive inventory and inefficiencies.
In medical writing, overproduction manifests as the generation of unnecessary or redundant content that adds little value to the reader. Over-documentation can clutter the workflow and overwhelm the audience.
Applying lean principles helps writers focus on creating only what is necessary, exactly when it’s needed. AuroraPrime Create promotes this with its lean summary generation feature, ensuring content is concise, relevant, and targeted to the audience’s needs.
Waiting
Waiting, another form of waste in manufacturing, occurs when processes are idle due to bottlenecks or downtime, delaying productivity.
In medical writing, this can take the form of delayed approvals, slow feedback cycles, or waiting for input from multiple stakeholders. These delays disrupt workflow continuity and slow the completion of projects.
To minimize waiting, lean principles focus on enhancing workflow efficiency. With real-time collaboration features, AuroraPrime Create enables teams to work together seamlessly, speeding up the review and approval process and reducing unnecessary delays.
Transport
Transport refers to the unnecessary movement of materials or products, which increases the risk of damage or loss and contributes no value to the process.
In medical writing, this waste appears when drafts are transferred across multiple platforms or teams without clear benefits, increasing the potential for errors and delays.
To reduce this waste, AuroraPrime Create integrates seamlessly with document management systems, streamlining transitions between writing stages and minimizing unnecessary transfers of content.
Overprocessing
Overprocessing occurs when more effort, time, or resources are used than necessary to complete a task.
In medical writing, overprocessing might involve over-explaining concepts, adding irrelevant details, or going through excessive review cycles. This leads to wasted time and effort that doesn’t enhance the document's value.
By focusing on essential content and efficient processes, teams can reduce overprocessing. AuroraPrime Create supports this with end-to-end automation for documents like protocols and clinical study reports (CSRs), eliminating redundant tasks and accelerating the overall writing process.
Inventory
Excess inventory in manufacturing refers to holding more materials or products than needed, tying up resources and potentially leading to obsolescence.
In medical writing, inventory waste arises from unused drafts, excessive notes, or unnecessary document versions. This clutter creates confusion and complicates the writing process.
AuroraPrime Create addresses this through content reuse and repurpose features, allowing writers to efficiently manage existing content, reduce the number of drafts, and ensure only relevant information is used.
Motion
In manufacturing, motion waste occurs when workers engage in unnecessary physical movement, which reduces efficiency.
In medical writing, this equates to inefficient workflows, such as searching for documents, switching between tools, or managing disorganized files. These inefficiencies reduce productivity and increase frustration.
With features like large-scale TFL incorporation, AuroraPrime Create minimizes unnecessary steps, allowing writers to quickly access and organize information, improving overall workflow efficiency.
Defects
Defects in manufacturing refer to errors or quality issues that result in rework, waste, or scrapping of products.
In medical writing, defects can include grammatical errors, incorrect data, or poorly structured content that requires extensive revisions. Ensuring high-quality, error-free documentation is essential for effective communication.
AuroraPrime Create offers several quality control features, including polish text, batch validate TFL summaries, and a quality control review process, all of which ensure content aligns with predefined standards. With QC mechanisms that incorporate a golden dataset and integrated writing instructions, the platform helps medical writers consistently produce high-quality, error-free documentation.
Conclusion
By identifying and eliminating these seven forms of waste, medical writers can significantly increase their efficiency and productivity. Combining lean principles with advanced tools like AuroraPrime Create leads to fewer errors, higher-quality documents, and streamlined processes, ultimately improving communication and contributing to better patient outcomes and faster clinical trials.





