Agile Intelligence: AuroraPrime RMA's LLM-Agnostic Design Empowers Your Scientific Future
Jul 30, 2025🚀 Agile Intelligence meets Scientific Precision 🔬What if your regulatory or medical authoring AI could think flexibly—adapting to any LLM, data environment, or scientific standard?Meet AuroraPrime RMA, AlphaLife’s LLM-agnostic AI engine that’s redefining content generation in life sciences. With modular plug-and-play capabilities, data provenance, and rigorous traceability, it’s built for the real complexity of global submissions and scientific documentation. 🌐📄
In the fast-evolving landscape of life sciences, staying ahead means embracing innovation without sacrificing control. At AlphaLife Sciences, our flagship AuroraPrime Generative AI platform, and specifically our AuroraPrime RMA (Regulatory and Medical Authoring) solution, embodies this principle perfectly. We understand that in a field as dynamic as ours, flexibility isn't just a nice-to-have; it's a strategic imperative. That's why AuroraPrime RMA is engineered with a cutting-edge LLM-agnostic design, offering unparalleled adaptability to meet your precise needs and future-proof your regulatory and medical writing workflows.
Beyond the Hype: True LLM Agnosticism
What does "LLM-agnostic" truly mean for your organization? It means AuroraPrime RMA isn't tied to a single large language model. Our platform is built on a modular AI and Large Language Model (LLM) framework, utilizing a cloud-agnostic, Kubernetes-based microservices architecture. This advanced design ensures high flexibility and configurability for diverse enterprise requirements, effortlessly adapting to new document types, therapeutic areas, and study phases.
This foundational flexibility allows AuroraPrime RMA to:
Seamlessly switch between different LLMs: Our model-agnostic architecture supports integration with a wide range of leading LLMs and can effortlessly connect with your client-specific AI endpoints. We support various engines, including but not limited to gpt-4o, gpt-4o-mini, o1, and o3-mini.
Leverage our Compose™ Low-Code Framework and AI & LLM Workbench: These tools provide a developer-friendly environment, simplifying the creation of compliance-ready applications. They enable the flexible adjustment of components like LLM models and prompt frameworks, directly addressing the need for adaptable AI-driven solutions.
Empower intelligent workflows with the Agent Framework: This framework allows for the creation of sophisticated AI agents by leveraging LLMs, defining their behavior, integrating them with Compose applications, and enhancing their capabilities with structured output (using JSON Schema) and knowledge retrieval via Retrieval-Augmented Generation (RAG).
Offer robust Prompt Management: You can configure and customize prompts at both system and workspace levels, with support for dynamic parameters to inject contextual information. This fine-tuned control over AI behavior ensures the generated content aligns perfectly with your requirements.
Crucially, AuroraPrime RMA maintains a human-in-the-loop AI interaction. Medical writers remain firmly in control, guiding specific tasks with an AI chat assistant, monitoring asynchronous AI progress, and most importantly, providing feedback on AI-generated content (e.g., "thumbs up" or "thumbs down" with categorized issues like "False Content" or "Irrelevant Content"). This feedback is not just collected; it's retained and used to continuously improve the AI's performance and ensure content accuracy and relevance.
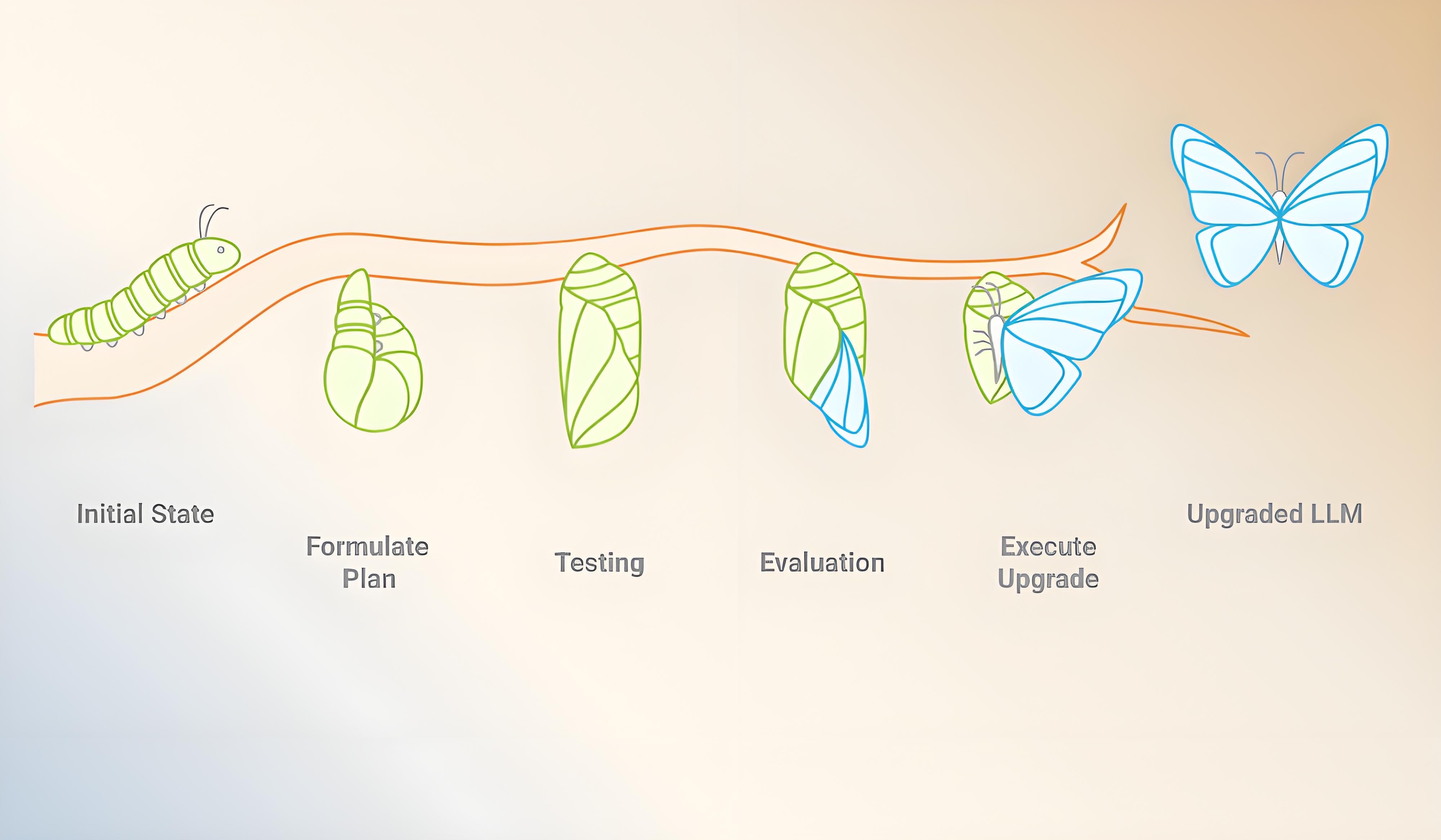
The AuroraPrime LLM Upgrade Workflow: Precision and Continuity
Adopting new LLMs or upgrading existing ones within AuroraPrime RMA is a structured and quality-controlled process, designed to ensure seamless integration, functional continuity, and improved performance. Our meticulous four-stage iterative workflow guarantees that your AI capabilities evolve without disruption:
Formulate Upgrade Plan: This initial stage involves defining the scope of the upgrade, whether it's switching to a new LLM version, updating prompt templates, or introducing new system logic. It outlines the target model version, associated prompt packages that need adaptation or replacement, and dependencies with the broader solution, such as document templates and QC workflows.
Testing: Once the plan is in place, we run automated regression tests to validate that existing capabilities remain stable with the new model. We also launch AI quality auto-evaluation jobs to rigorously test core functions, including content accuracy, consistency, template adherence, and handling of edge cases and critical prompts. These tests are vital for ensuring backward compatibility and surfacing any early signals of behavioral drift or degradation. AlphaLife Sciences also utilizes "golden test sets" to regularly monitor and assess AI-generated content quality when the model is upgraded or changed.
Evaluation: This phase involves a comprehensive quality assessment. We combine quantitative metrics from auto-evaluations (e.g., BLEU scores, factual accuracy benchmarks) with human evaluation to verify content relevance, tone, and scientific soundness in real-world use cases. Results are compared against pre-upgrade baselines to confirm improvement or identify any regressions.
Execute Upgrade Plan: Upon successful evaluation, the new model and its associated prompt package are deployed into the production environment. Every step of the implementation is meticulously documented, and a detailed upgrade report is compiled, covering regression outcomes, evaluation findings, and rollback procedures (if applicable).
This systematic approach ensures that AuroraPrime RMA remains at the forefront of AI advancements, rapidly adopting the latest models and technologies to continuously enhance its capabilities while maintaining the highest standards of quality and reliability.
By offering this level of control, adaptability, and a clear path for technological evolution, AuroraPrime RMA empowers life sciences organizations to accelerate their drug development timelines and achieve transformative outcomes with confidence. It's not just about automating tasks; it's about intelligent, flexible partnership in your journey to scientific breakthrough.





