Beyond the Hype Cycle: Why LLM Agnosticism is the Foundation of Future-Proof Regulatory Strategy
Nov 05, 2025🚀 The AI revolution in life sciences is accelerating—but how do we separate genuine transformation from fleeting hype? At AlphaLife Sciences, we believe the answer lies in LLM agnosticism—a strategy that goes beyond model loyalty to deliver sustainable, future-proof innovation.💡 By staying flexible, interoperable, and evidence-driven, organizations can harness the full potential of AI to streamline regulatory, medical, and clinical workflows—without being locked into a single tech ecosystem.🌍 In a world where technology evolves faster than regulation, adaptability isn’t optional—it’s a competitive advantage.
In the rapidly evolving world of Generative AI (GenAI), change is the only constant. New Large Language Models (LLMs) are launched seemingly every quarter, each promising better performance, efficiency, or domain specialization. For the life sciences industry, which demands stability, rigor, and compliance, this volatility presents a challenge: How do you invest heavily in AI without risking immediate obsolescence or crippling vendor lock-in?
At AlphaLife Sciences, we engineered the answer into the very foundation of our flagship AuroraPrime RMA (Regulatory and Medical Authoring) platform. Our solution is built not on a single LLM, but on an LLM-agnostic architecture. This strategic choice provides our clients with the flexibility needed to harness AI innovation while maintaining the continuous production and quality control necessary for high-stakes documents like Clinical Study Reports (CSRs), Development Safety Update Reports (DSURs), and Patient Safety Narratives.
The Freedom to Choose: Decoupling Application from Algorithm
Imagine building a multi-million-dollar drug manufacturing facility designed to run exclusively on one type of motor that could be discontinued next year. That risk is unacceptable in pharma R&D. The same logic applies to core regulatory technology.
AuroraPrime RMA ensures that our application logic—the purpose-built framework that slashes CSR first draft time by 90%—is completely decoupled from the underlying AI engine.
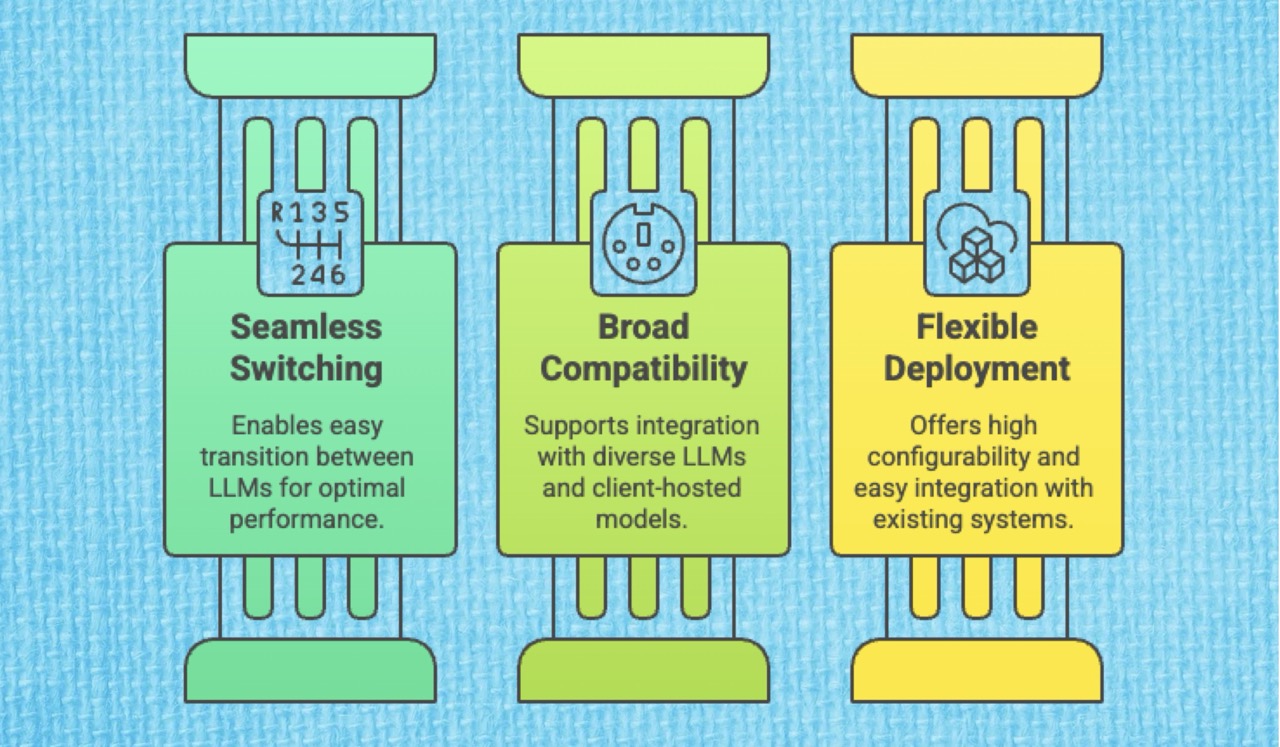
This LLM-agnostic architecture offers several crucial strategic advantages to global pharmaceutical enterprises:
Seamless Switching: The platform has built-in capabilities for seamlessly switching between different LLMs. If a new model proves superior for highly complex tasks, such as generating Summaries of Clinical Safety and Efficacy (CTD Module 2.5 and 2.7), clients can easily adapt their content generation capabilities.
Broad Compatibility: AuroraPrime supports integration with a wide array of leading LLMs, including gpt-4.1, Gemini, Claude, and Llama. It also offers the flexibility to integrate seamlessly with client-hosted AI models, aligning perfectly with internal IT security and data strategies.
Flexible Deployment: Our underlying modular AI and Large Language Model (LLM) framework, combined with a low-code architecture, ensures high flexibility and configurability. Furthermore, if a client’s platform API is compatible with the OpenAI API specification, integration can be achieved simply by updating the endpoint configuration.
By embracing this flexibility, AuroraPrime RMA ensures that clients can establish a scalable content generation capability that is prepared for future R&D use cases and technological evolution.
Engineered for Perpetual Optimization, Not Interruption
Transitioning to a new technology model traditionally involves high costs, downtime, and extensive validation. AuroraPrime transforms this process through a structured, quality-controlled approach for evolving the LLM strategy.
Rather than relying on continuous model fine-tuning or altering base model weights—which can compromise stability—AuroraPrime enhances performance through contextual engineering and advanced memory techniques. We rely heavily on Retrieval-Augmented Generation (RAG) and continuous user feedback to refine prompt instructions and integrate domain knowledge.
When a switch or upgrade of the core LLM is required, we follow a rigorous, four-stage process to ensure seamless functional continuity:
Formulate Upgrade Plan: This stage defines the upgrade scope, outlining the target model version (e.g., GPT-4 to GPT-4 o) and identifying the associated prompt packages and dependencies that need to be adapted or replaced.
Testing: We run automated regression tests to validate that existing capabilities remain stable. Crucially, we launch AI quality auto-evaluation jobs that test core functions for content accuracy and consistency, ensuring backward compatibility.
Evaluation: A comprehensive quality assessment is performed, combining quantitative metrics (like factual accuracy benchmarks) with human evaluation. This human review verifies crucial elements like content relevance, tone, and scientific soundness in real-world scenarios.
Execute Upgrade Plan: Upon successful evaluation, the new model is deployed, and a detailed upgrade report is compiled, covering all regression outcomes and findings.
This rigorous, documented process provides the necessary transparency, traceability, and compliance required by the pharmaceutical industry.
Total Control and Uncompromising Security
For life sciences, data integrity and control are non-negotiable. Our LLM-agnostic design is integral to our security framework:
Data Isolation: Client data is never used for model training, ensuring strict data segregation and security. Data remains isolated within each client environment.
Enterprise Integration: The architecture allows for effortless connection with client-specific AI endpoints.
By adopting AuroraPrime RMA, you are choosing a production-proven solution trusted by 5 out of the top 10 global pharma companies that gives you ultimate control over your R&D documentation destiny. We ensure that your investment in GenAI remains valuable today, tomorrow, and well into the future, accelerating the delivery of life-changing treatments to patients worldwide.





