Beyond the Keyboard: How AI Co-Piloting Unleashes the Strategic Power of Medical Writers
Oct 10, 2025🚀 What if AI wasn’t just a tool, but a true co-pilot for medical writers—lifting the heavy drafting load so experts can focus on what matters most: insight, impact, and innovation? In our latest article, we explore how AuroraPrime transforms medical writing by automating the routine and enabling writers to shift into strategic mode. Dive in and see how AI + human expertise combine to advance life sciences writing beyond the keyboard. ✍️🔬
In the intricate world of life sciences, medical writers play an indispensable role, translating complex scientific data into clear, compliant, and impactful documents. Yet, this vital function often grapples with the sheer volume of content creation, repetitive tasks, and the constant pressure of tight deadlines and evolving regulatory landscapes. It's a field ripe for transformation, and at AlphaLife Sciences, we believe the key lies not in replacing human expertise, but in empowering it.
This is precisely where AuroraPrime steps in – not as a replacement for the skilled medical writer, but as an intelligent co-pilot, revolutionizing medical and regulatory writing through intelligent automation and digital innovation. Our mission is to be the leading generative AI-powered digital solution enabler for life sciences, driving productivity and quality throughout the drug lifecycle.
AuroraPrime: Your AI Co-Pilot in Action
AuroraPrime is a next-generation, generative AI-powered platform specifically designed for content authoring and documentation in the life sciences sector. It’s built on a modular AI and LLM framework that flexibly integrates with existing enterprise systems, streamlining the entire product development lifecycle. More than just a tool, AuroraPrime augments, rather than replaces, human expertise, ensuring medical writers remain in control of the end-to-end process.
So, how does this AI co-pilot empower medical writers to unleash their strategic potential? By taking on the heavy lifting of routine and time-consuming tasks:
1. Accelerating Initial Draft Generation with Unprecedented Speed
Imagine drastically cutting down the time spent on drafting complex documents. AuroraPrime automates the end-to-end creation of critical documents like:
Clinical Study Reports (CSRs)
Protocols
Safety Narratives (including Patient Safety Narratives)
Development Safety Update Reports (DSURs) and Periodic Safety Update Reports (PSURs)
Investigator's Brochures (IBs)
CTD Modules (e.g., 2.5 Clinical Overview, 2.7.3 Summary of Clinical Efficacy, 2.7.4 Summary of Clinical Safety)
Lay Summaries
The impact is significant: industry leaders using AuroraPrime have reported a 90% reduction in first draft time for CSRs and Protocols, and a 95% reduction for Safety Narratives. This means tasks that traditionally took weeks can now be completed within minutes. This automation is achieved through AI-driven drafting, updates that synthesize upstream documents and data, and the use of flexible, pre-built templates.
2. Mastering Tables, Figures, and Listings (TFLs)
TFLs are central to clinical documentation, but their integration and summarization are often laborious. AuroraPrime streamlines this process with large-scale TFL automation:
Automated TFL Summary Generation: The platform automatically generates summaries of TFLs using AI, with options for custom prompts and data filtering to enhance speed and accuracy.
Batch Processing: TFL summaries can be batch generated, updated, and validated asynchronously, freeing writers to focus on other tasks.
Seamless Integration: It manages and inserts TFLs into documents more efficiently, including smart matching to source data and importing from Excel.
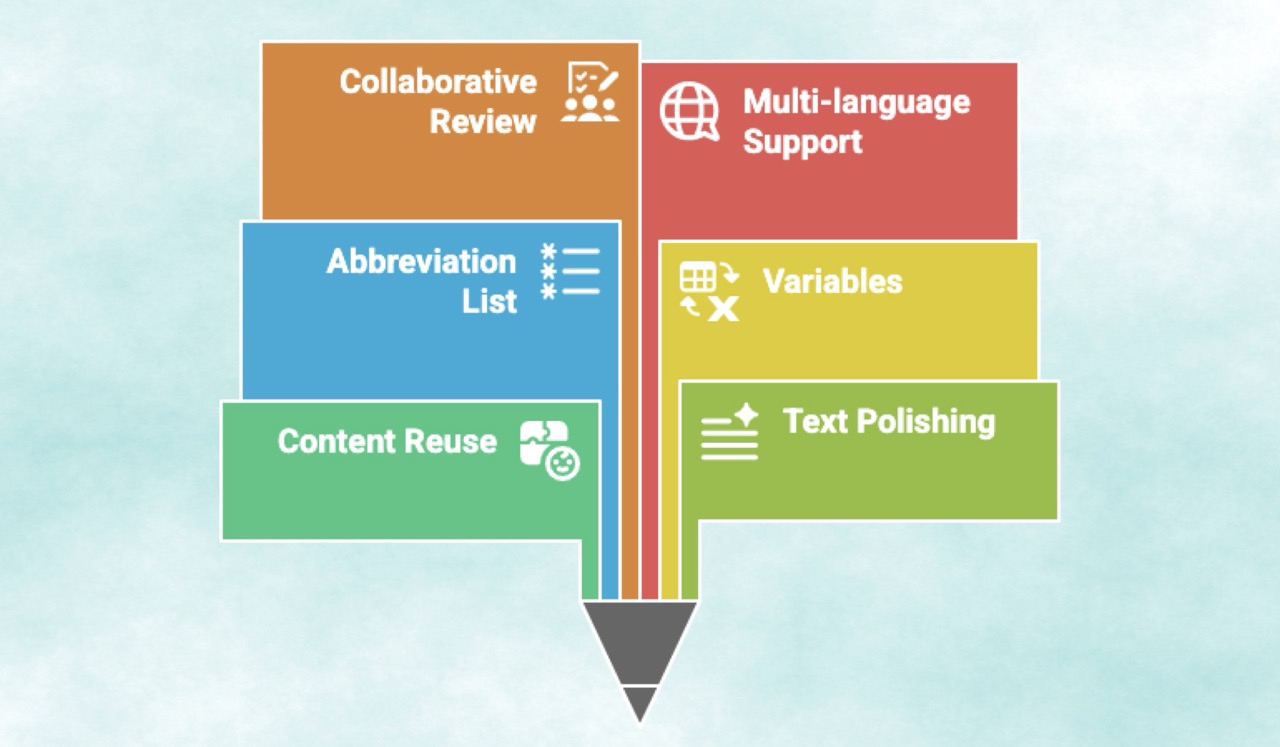
3. Streamlining Editing, Review, and Finalization
Beyond drafting, AuroraPrime provides a comprehensive suite of tools to refine and finalize documents:
Content Reuse: It enables efficient content reuse from existing documents for maximum productivity and consistency.
Text Polishing and Tense Conversion: Tools are available to polish text for clarity and professionalism, and even convert verbs to past tense.
Abbreviation List Creation: It automates the incorporation of abbreviation lists with flexible, customizable rules.
Variables: Writers can work with variables to manage and update recurring information efficiently, ensuring uniformity and reducing errors.
Collaborative Review & Version Control: The platform supports efficient review and refinement of drafts while maintaining alignment throughout the process, leveraging native Microsoft Word capabilities for version control.
Multi-language Support: AuroraPrime supports the creation and translation of clinical documentation into multiple languages.
All these AI tasks, such as translation, polishing, and summary generation, are processed asynchronously in the background, allowing medical writers to continue editing other sections of the document without waiting for tasks to complete. This significantly enhances overall productivity.
Focusing on What Truly Matters: Strategic and High-Value Activities
By offloading repetitive and time-consuming tasks, AuroraPrime empowers medical writers to shift their focus to higher-value activities that truly leverage their expertise and strategic thinking:
Scientific Quality and Strategic Review: Instead of manual formatting and data transcription, writers can delve deeper into the scientific accuracy, interpretation, and strategic implications of the data.
Complex Problem-Solving: With routine tasks automated, writers can dedicate more time to intricate challenges, ensuring the narrative effectively communicates nuanced findings.
Innovation: Free from mundane duties, R&D leaders and medical writers can concentrate on exploring new research avenues and driving genuine innovation in drug development.
Compliance and Factualness: While AuroraPrime has built-in GenAI QC and performs automated factualness checking by benchmarking against "golden" standards and comparing summary text against TFL data, human oversight remains paramount for final validation and ensuring precision. This allows writers to focus on the nuanced application of regulatory intelligence and complex compliance issues.
Built for Trust: Compliance and Integration
AuroraPrime operates directly within a familiar environment: Microsoft Word 365. This seamless integration ensures a continuous workflow without needing to switch applications. Furthermore, our platform prioritizes robust quality governance and adheres to stringent global regulatory standards, including FDA 21 CFR Part 11, HIPAA, GDPR, ISO 9001, ISO 27001, and AICPA SOC 2 Type II. We also incorporate feedback mechanisms (thumbs up/down) to continuously improve the AI's performance and ensure content accuracy and relevance.
Proven Impact: Real Results in the Real World
AuroraPrime is not just a concept; it has a proven track record of success with global pharmaceutical leaders. It has been adopted by 5 out of the top 10 global pharma companies and several leading Contract Research Organizations (CROs).
A Top 5 Global Pharmaceutical Company affirmed, "AlphaLife Sciences sets the industry standard. Your innovative solutions are transformative—enhancing efficiency and quality across our operations".
A Top 10 Global Pharmaceutical Company confirmed a 30% time-saving through a pilot using historical CSRs, allowing them to "focus more on strategic tasks while maintaining high-quality outputs".
A Top 3 Global Leading CRO highlighted significant efficiency gains, "particularly in content reuse," streamlining workflows for CSRs, SAPs, and study protocols, thereby "saving time and reducing redundancy".
Beyond these efficiency gains, AuroraPrime projects significant revenue acceleration potential for leading pharmaceutical companies, such as $1.4 billion in revenue acceleration and 3.5% annual revenue growth for a Top 20 Global Pharma.
By transforming the arduous task of medical writing, AuroraPrime enables life sciences organizations to accelerate drug development timelines and bring life-saving treatments to patients faster. It's about maximizing human potential, turning writers into strategic architects of information, and accelerating the journey from scientific discovery to patient care.
Ready to empower your medical writing team and accelerate your drug development pipeline? Book a demo with AlphaLife Sciences today.





