Breaking Down Silos—Fostering Seamless Collaboration in Clinical Trials with AuroraPrime Create
Dec 03, 2024🚀 In the fast-paced world of clinical research, collaboration is key to innovation. But how can we overcome silos that hinder progress? 🤔 Discover how AuroraPrime Create is revolutionizing teamwork, enabling seamless collaboration across teams, and accelerating breakthroughs in healthcare. 💡
Clinical trials are intricate undertakings involving multiple stakeholders, each generating vast amounts of data. This complexity often leads to information silos that hinder effective communication and collaboration across teams. AuroraPrime Create, a robust Microsoft Word add-in from AlphaLife Sciences, addresses this challenge by providing a centralized platform that streamlines workflows and enhances data transparency throughout the clinical trial process.
Breaking Down Information Silos with AuroraPrime Create
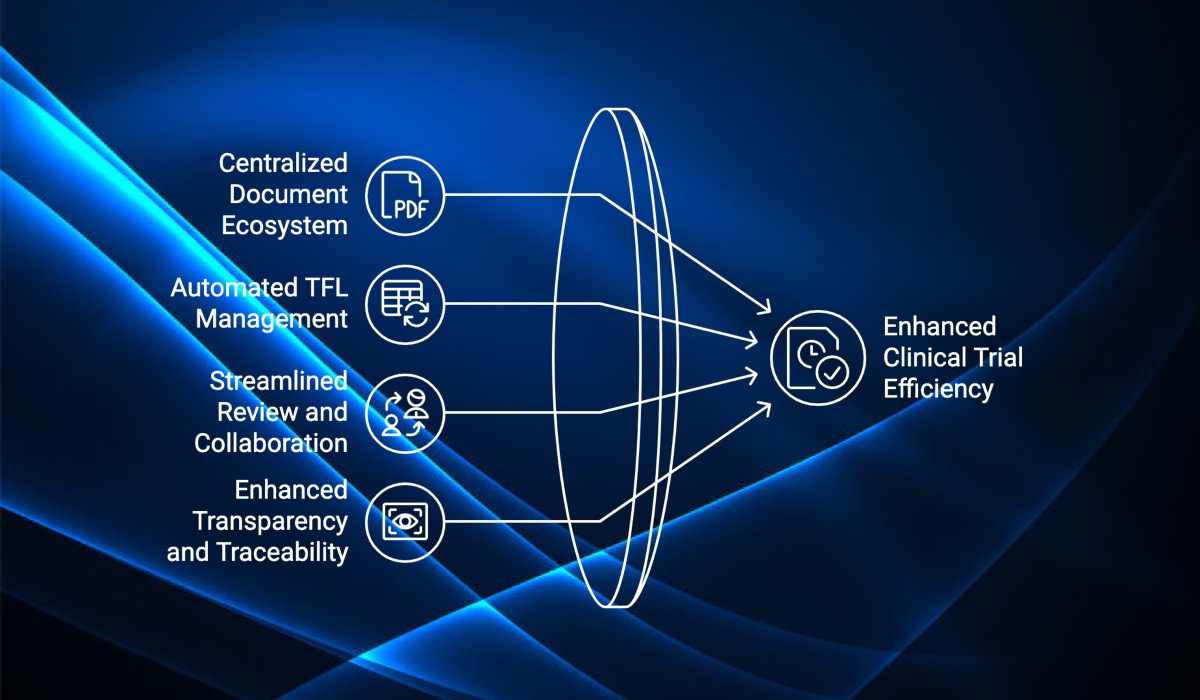
Centralized Document Ecosystem: AuroraPrime Create integrates seamlessly with Microsoft 365, RIM systems, and review platforms, creating a unified ecosystem for clinical trial documents. Stakeholders can access, share, and contribute to essential documents in real-time, eliminating the need for separate repositories and fostering collaboration among medical writers, biostatisticians, clinical research teams, and more.
Automated TFL Management: The process of adding Tables, Figures, and Listings (TFLs) to clinical study reports (CSRs) often requires extensive coordination between medical writers and statistical programmers. AuroraPrime Create automates TFL insertion and updates, ensuring that all team members are working with the latest data and reducing communication bottlenecks.
Streamlined Review and Collaboration: Effective collaboration is essential in regulatory writing. AuroraPrime Create facilitates this with built-in tools for document review, annotation, and version control, allowing teams to provide feedback, track changes, and stay aligned on document content.
Enhanced Transparency and Traceability: By centralizing document-related activities within a secure platform, AuroraPrime Create improves transparency and traceability throughout the clinical trial process. It simplifies document history tracking, contributor identification, and compliance with regulatory standards.
Enabling a Collaborative Approach to Clinical Trial Documentation
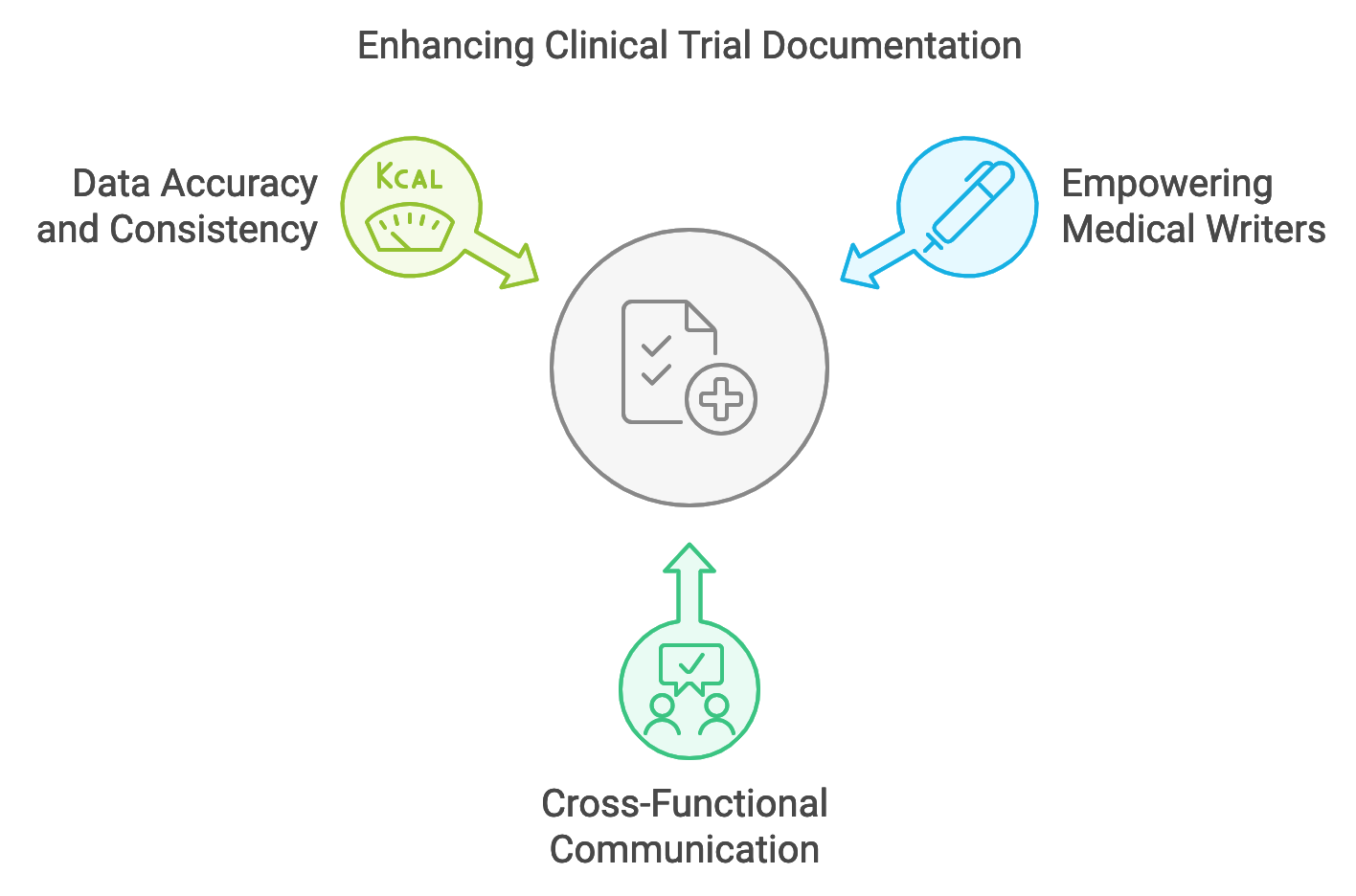
Empowering Medical Writers: AuroraPrime Create empowers medical writers to take an active role in the clinical trial process. With automated TFL management and streamlined content generation, writers can focus on higher-level tasks such as data analysis and ensuring document clarity and accuracy.
Fostering Cross-Functional Communication: AuroraPrime Create’s collaborative features dismantle communication barriers between functional teams like medical writing, biostatistics, and clinical research. This integrated approach ensures that diverse perspectives are included in document creation.
Improving Data Accuracy and Consistency: By automating data-driven tasks and centralizing document management, AuroraPrime Create helps minimize errors and ensures data consistency across all clinical trial documentation.
AuroraPrime Create: A Catalyst for a More Collaborative Future
As the demand for skilled regulatory writers grows, AuroraPrime Create equips them with essential tools to navigate the complexities of drug development. By dismantling information silos and fostering seamless collaboration, AuroraPrime Create enhances efficiency, data accuracy, and a more integrated approach to clinical trial documentation—ultimately contributing to safer and more effective patient treatments.





