Built for Growth: How AuroraPrime's AI Scales and Adapts to Your Life Sciences Vision
Aug 18, 2025🚀 Ready to future-proof your life sciences documentation? Discover how AuroraPrime RMA, the AI-powered platform from AlphaLife Sciences, is built for scalability, customizability, and regulatory precision—helping medical writers everywhere turn weeks of work into minutes. From dynamic templating and human-in-the-loop refinement to multi-LLM support, modular architecture, and enterprise-grade compliance—this is AI that truly grows with your R\&D vision.
In the rapidly evolving landscape of life sciences, innovation isn't just about groundbreaking therapies; it's also about optimizing the very processes that bring them to light. Clinical documentation, the bedrock of regulatory submissions and scientific integrity, is a prime example. For too long, its inherent complexities have presented a dilemma: how do you ensure rigorous quality and compliance without sacrificing agility and the ability to grow?
At AlphaLife Sciences, we believe the answer lies in intelligent design. Our flagship AuroraPrime Generative AI platform and its AuroraPrime RMA (Regulatory and Medical Authoring) solution are engineered from the ground up not just to automate, but to be profoundly scalable and customizable, ensuring your R&D efforts are future-proof and hyper-efficient.
Let's delve into how AuroraPrime empowers you with unparalleled flexibility and the capacity to expand.
The Power of Adaptability: Customization at Its Core
No two life science organizations are identical, nor are their documentation needs. AuroraPrime RMA embraces this reality, offering a highly configurable platform that molds to your unique workflows and requirements.
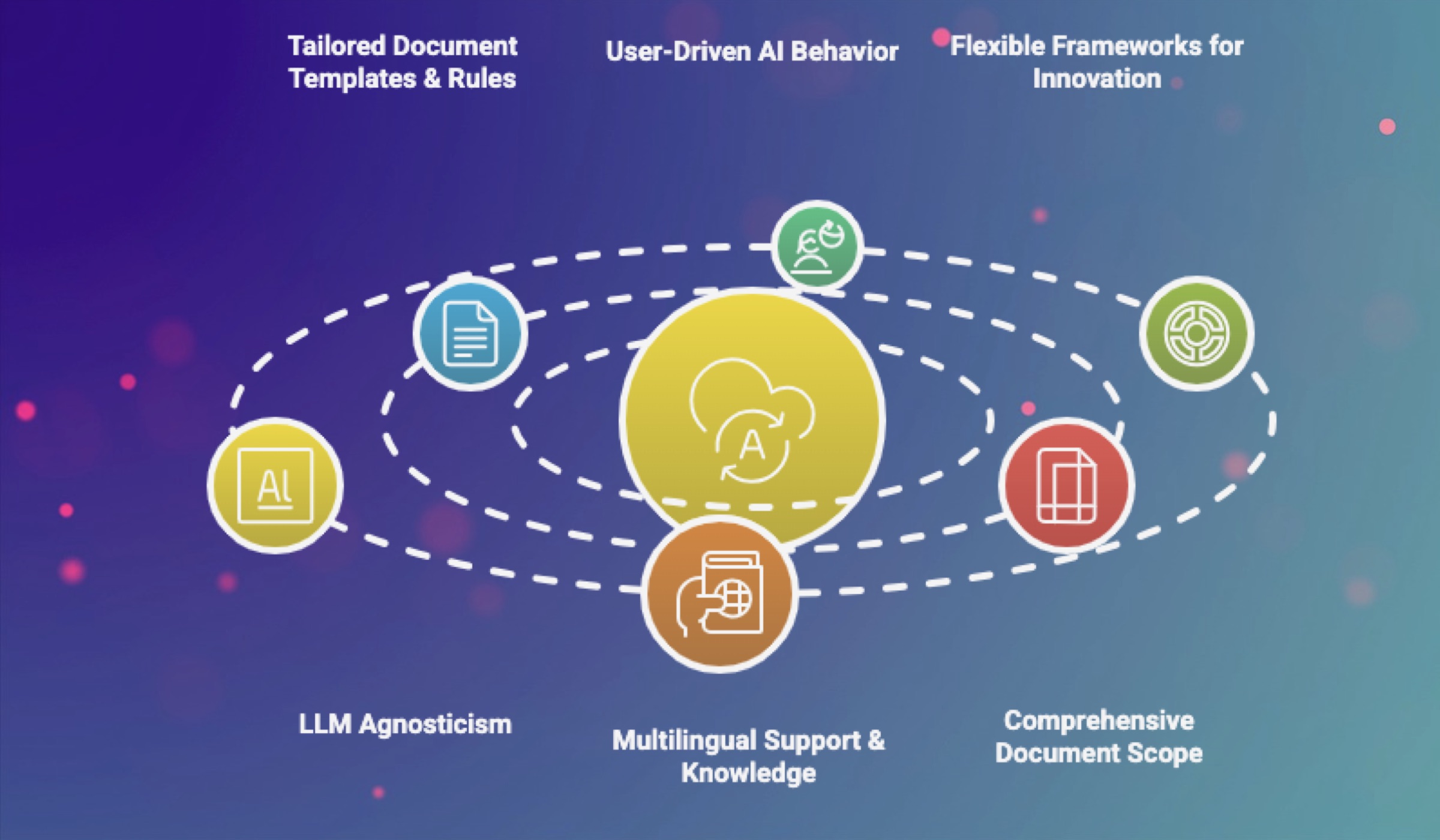
Tailored Document Templates & Rules: AuroraPrime RMA enables Template Admins to easily create and configure AI-enabled document templates directly from existing Word documents. You can define precise content generation rules for each section, specifying information sources and guiding the AI on how to auto-generate content. These intelligent templates allow for granular control over document generation.
User-Driven AI Behavior: Medical writers retain human-in-the-loop (HITL) control, interacting with an AI chat assistant to guide specific tasks and provide feedback on AI-generated content, which continuously improves the AI's performance. The platform supports user-interactive prompt tuning and a robust Prompt Management system for configuring and customizing prompts at both system and workspace levels, including dynamic parameters. Writers can even define custom content generation rules directly within the document writing mode for ad-hoc content.
Flexible Frameworks for Innovation: Built on a modular architecture and the Compose™ Low-Code Framework, AuroraPrime offers ultimate flexibility and configurability. This enables rapid development and adaptation for new document types, therapeutic areas, and study phases. It features an AI & LLM Workbench that simplifies building compliance-ready applications.
LLM Agnosticism: AuroraPrime RMA supports seamlessly switching between different Large Language Models (LLMs), including GPT 4o, GPT-4, GPT-3.5, CHAT_GLM_4, and MOONSHOT, allowing you to leverage the best-suited AI for specific tasks and evolve with cutting-edge advancements. The Agent Framework further allows for the creation of intelligent workflows, structured output using JSON Schema, and knowledge retrieval via Retrieval-Augmented Generation (RAG).
Multilingual Support & Knowledge: The platform provides multilingual document support, including AI-powered translation solutions for regulatory documents and the ability to define organization-level translation dictionaries. It also includes extensible, case-specific knowledge bases (Cyclopedia™) adaptable to different therapeutic areas and study designs, which can automatically integrate regulatory changes into workflows.
Comprehensive Document Scope: AuroraPrime supports a comprehensive and evolving range of clinical and regulatory documents, including CSRs, Protocols, Patient Safety Narratives, DSURs, PSURs, Clinical Overviews (Module 2.5), Summaries of Clinical Efficacy (Module 2.7.3), and Summaries of Clinical Safety (Module 2.7.4), among others.
Scaling New Heights: Building for Enterprise Growth
AuroraPrime is not just about individual document creation; it’s designed for enterprise-level deployment and growth, capable of handling extensive documentation needs across a large organization.
Cloud-Native and Modular Architecture: AuroraPrime is a cloud-agnostic, Kubernetes-based microservices architecture, designed for modular deployment across diverse environments like AWS. It's delivered as a Platform-as-a-Service (PaaS), leveraging secure and scalable cloud infrastructure like Microsoft Azure to ensure robust and highly available solutions. This design delivers agility, efficiency, and security for rapid time-to-value.
Robust Data Handling & Integration: The platform offers multimodal data ingestion capabilities, seamlessly incorporating complex tabular data like TFLs (Tables, Figures, and Listings) from RTF and Excel files, and Schedules of Assessments (SOAs). It integrates effortlessly with existing enterprise systems such as Microsoft Word, Veeva Vault/RIM, Electronic Data Capture (EDC) platforms, and Pharmacovigilance (PV) systems. AlphaLife Sciences' participation in the Veeva AI Partner Program ensures deep API connectivity for efficient data extraction and streamlined file management.
High Performance and Efficiency at Scale: AuroraPrime RMA is engineered for high concurrency and robustness, with safeguards at three levels including automatic retries for LLM interactions and real-time monitoring. It automates initial drafts of critical documents with 90% reduction in first draft time for CSRs and Protocols, and 50-70% overall time reduction for these documents, turning weeks into minutes. It supports batch generation of study protocol section content and batch TFL incorporation, significantly accelerating workflows. Time-consuming AI tasks are processed asynchronously in the background, allowing writers to continue other work uninterrupted.
Proven Success with Industry Leaders: AuroraPrime is a platform proven in global pharmaceutical deployments, adopted by five out of the top 10 global pharmaceutical companies and leading Contract Research Organizations (CROs). Clients are experiencing significant efficiency gains, with one top-10 global pharmaceutical company confirming a 30% time-saving in their Medical Writing Team through a pilot. Another top-3 global CRO highlighted significant efficiency gains, particularly in content reuse, streamlining workflows for CSRs, SAPs, and study protocols. Johnson & Johnson (J&J) is significantly expanding its use of AuroraPrime, planning to go to full scale with over a hundred CSRs in the subsequent year.
Rigorous Compliance Foundation: The platform adheres to rigorous global regulatory standards, including FDA 21 CFR Part 11, HIPAA, GDPR, ISO 9001, ISO 27001, and AICPA SOC 2 Type II, and is capable of generating and performing quality control of GxP reports automatically. This foundational compliance ensures the solution meets the rigorous demands of the life sciences industry for secure and trustworthy data handling.
The AuroraPrime Advantage: Future-Proofing Your R&D
By combining deep customizability with robust scalability, AuroraPrime RMA isn't just a tool; it's a strategic partner for life sciences organizations. It liberates your medical writers and R&D leaders from repetitive tasks, allowing them to focus on higher-value activities such as strategic planning, scientific quality, and core innovation. This comprehensive solution empowers teams to produce high-quality clinical documentation more efficiently, consistently, and with greater accuracy, ultimately accelerating drug development timelines and driving unparalleled success in bringing life-saving therapies to patients.
Ready to unlock new levels of efficiency and adaptability in your clinical documentation?
Contact AlphaLife Sciences today to learn more about AuroraPrime RMA and schedule a personalized demo.





