Compliance From Conception: Embedding Regulatory Intelligence Directly into AI Authoring with AuroraPrime RMA
Jan 14, 2026In the fast-paced world of life sciences, treating compliance as a final checkbox is a strategy of the past. Why wait until the end of a drafting cycle to discover a regulatory deviation? True acceleration happens when compliance is baked into the process from the very first keystroke. 🚀At AlphaLife Sciences, we are redefining the standard with AuroraPrime RMA. By embedding regulatory intelligence directly into AI-driven authoring, we are moving from reactive corrections to proactive precision. Imagine generating Clinical Study Reports and Protocols that align with global guidelines like ICH, FDA, and EMA from the moment of conception. 🌍✨
In the life sciences industry, compliance isn't a final check—it must be baked into the process from the very first draft. The regulatory landscape is complex, constantly shifting, and demanding. Relying on manual document reviews, outdated checklists, or human memory to ensure adherence to global guidelines (like ICH, FDA, and EMA) is a slow, high-risk proposition that costs pharmaceutical companies time and resources.
At AlphaLife Sciences, we engineered our flagship AuroraPrime RMA (Regulatory and Medical Authoring) platform to redefine this workflow. We ensure Compliance Assurance by embedding regulatory intelligence directly into the writing process, turning compliance from a burdensome final hurdle into a seamless, automated standard.
The Pitfall of Post-Draft Compliance
Historically, regulatory intelligence was applied after the document was drafted. A medical writer would complete a Clinical Study Report (CSR) or a Protocol, and then a compliance expert or quality control team would flag deviations against internal templates, ICH M4 requirements, or new safety reporting guidelines. This often triggered frustrating, expensive cycles of revision.
To truly accelerate drug development, regulatory documents—such as CSRs, Protocols, and Development Safety Update Reports (DSURs)—must meet required guidelines from the moment the AI begins drafting. AuroraPrime RMA makes this possible by streamlining end-to-end compliance.
Compliance by Design: RAG and Embedded Intelligence
AuroraPrime RMA simplifies compliance by leveraging leading AI technology, specifically Retrieval-Augmented Generation (RAG). RAG is the mechanism that allows our generative AI platform to access a specialized, internal knowledge library, ensuring that every piece of content generated is grounded in verified, authoritative sources—not just generalized internet knowledge.
This verified knowledge includes:
Standardized Knowledge and Terms: The platform utilizes Cyclopedia™ Knowledge Bases for standardized knowledge and terms, simplifying compliance by keeping processes aligned with regulations.
Embedded Regulatory Guidelines: AuroraPrime RMA embeds regulatory intelligence into the writing process, ensuring that every draft section adheres to current global guidelines, industry standards, and company-specific rules. This helps minimize compliance risks and ensures accurate submissions.
Historical Company Knowledge: Our centralized document repository, AuroraPrime DocMind, acts as a corporate AI knowledge base for storing documents like Health Authority (HA) questions and responses. This historical content can be efficiently retrieved and reused for future regulatory submissions, reducing repetitive manual work.
When the AI drafts a new safety section in a DSUR, for instance, it pulls the most relevant, pre-approved language from the corporate knowledge base via RAG, ensuring the content is scientifically accurate and regulatorily aligned.
Rigorous Quality Control and Auditability
Compliance assurance extends far beyond the initial drafting. AuroraPrime ensures rigor throughout the content lifecycle:
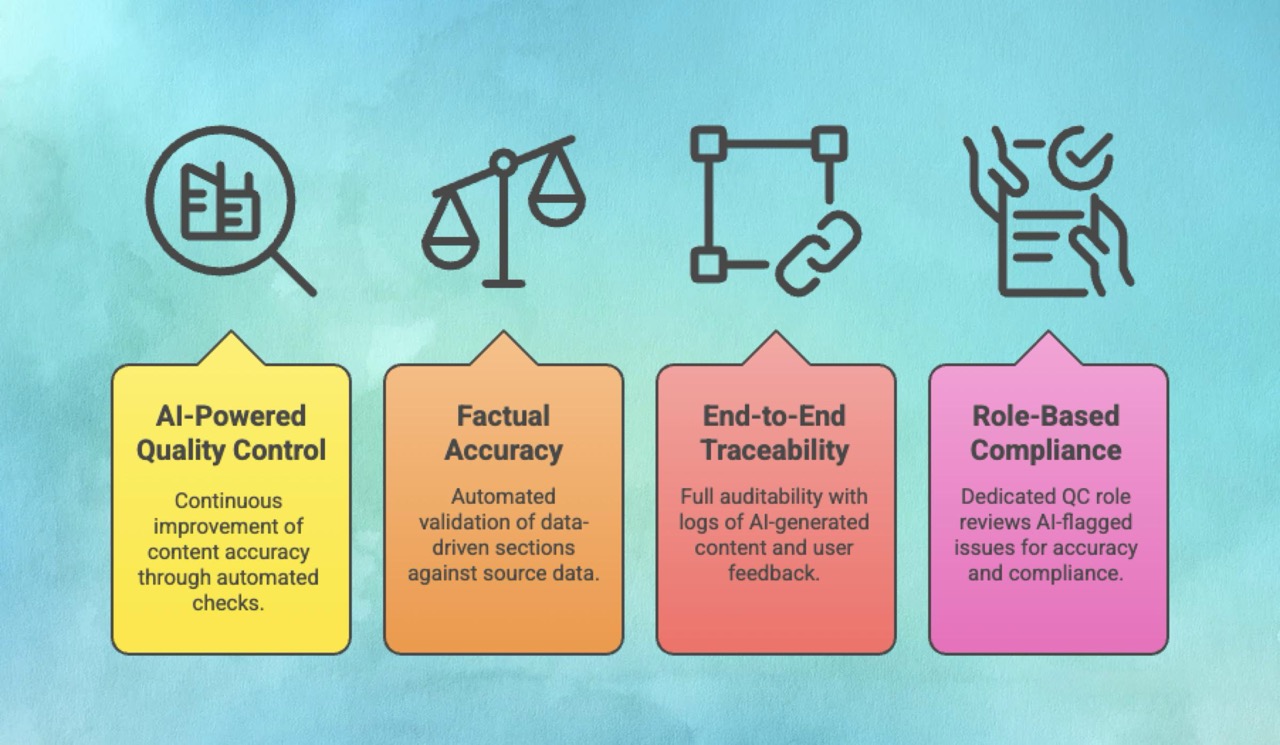
Built-in GenAI Quality Control (QC): The platform uses an AI-powered quality control framework that continuously improves accuracy and consistency. This QC system benchmarks generated content against "golden" standards, performing automated integrity and consistency checks to validate the logical flow and consistency of the document against regulatory requirements.
Factual Accuracy and Validation: For data-driven sections, such as TFL summaries in CSRs, the "Validate TFL Summaries" feature automatically compares the generated text against the source TFL data, flagging discrepancies and ensuring factual integrity.
End-to-End Traceability: In a regulated environment, auditability is essential. AuroraPrime RMA ensures transparency by meticulously logging all AI-generated content, user feedback, and edits within the AI Tasks pane, creating a clear audit trail. Furthermore, when content is reused from existing documents, the platform displays the original source reference directly beneath the segment, making verification easy.
Role-Based Compliance: The system includes a QC role responsible for reviewing and validating AI-flagged issues within documents, ensuring accuracy and compliance before finalization.
A Unified, Compliant Solution for Accelerating R&D
By automating regulatory adherence and building quality control directly into the drafting process, AuroraPrime RMA helps pharmaceutical companies achieve transformative outcomes. This unified, AI-powered platform streamlines document writing and generation, accelerating compliance and research efficiency.
AuroraPrime RMA adheres to globally recognized compliance standards, including FDA 21 CFR Part 11, HIPAA, and GDPR. By ensuring that every step, from initial template configuration to final quality check, is governed by embedded regulatory intelligence, AlphaLife Sciences empowers R&D leaders to focus on innovation while maintaining the highest level of regulatory confidence.
AuroraPrime RMA is the definitive AI-powered platform for clinical documentation, delivering speed, quality, and robust compliance to the world’s leading pharmaceutical companies.





