From Tool to Teammate: Why McKinsey’s Vision of "Agentic AI" is the Wake-Up Call Pharma Needs
Feb 04, 2026🤖➡️🤝 What happens when AI stops being just a tool—and starts acting like a true teammate?McKinsey’s vision of agentic AI signals a fundamental shift for pharma and life sciences: from automation that assists tasks to intelligent agents that reason, collaborate, and drive outcomes across regulatory, medical, and clinical workflows. This isn’t a future-state thought experiment—it’s a wake-up call for how work gets done, how decisions are made, and how organizations stay competitive.At AlphaLife Sciences, we see agentic AI as the catalyst for a new operating model: one where scientific expertise is amplified, document lifecycles are orchestrated end to end, and teams move faster without sacrificing rigor or compliance. The question is no longer if AI belongs at the table—but whether your organization is ready to work alongside it.
If you’ve been following the conversation around Artificial Intelligence in life sciences, you know the buzz has shifted. We are no longer just talking about ChatGPT writing funny emails or summarizing meeting notes. We are talking about a fundamental restructuring of how work gets done.
In their January 2026 issue, McKinsey on Life Sciences, the authors lay out a bold new reality: we are entering the era of Agentic AI.
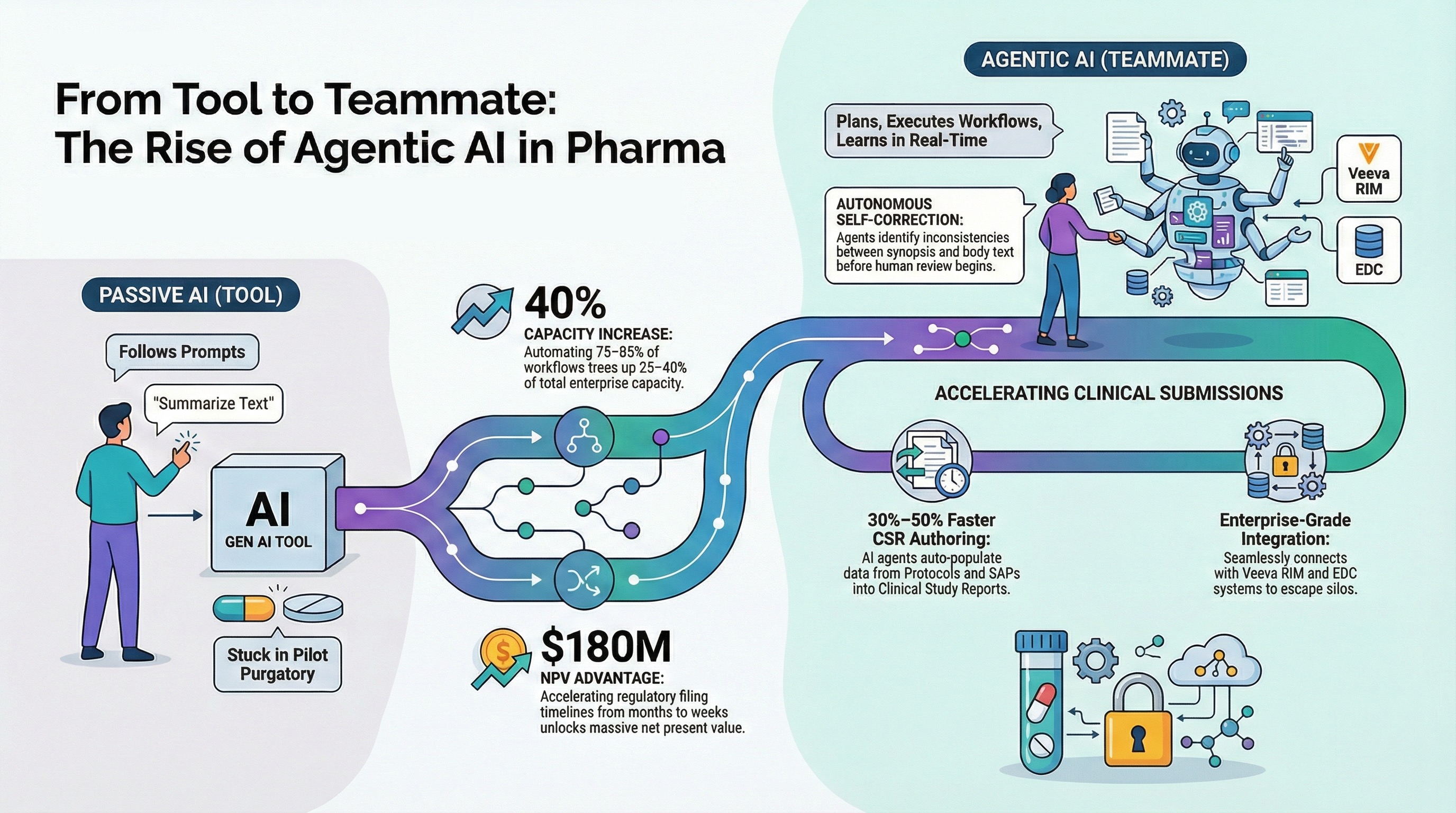
The distinction is critical. Traditional AI is a tool you wield—like a calculator or a spellchecker. Agentic AI, however, behaves more like a "coworker". It doesn't just wait for a prompt; it plans, executes workflows, interacts with other systems, and learns in real-time.
At AlphaLife Sciences, this resonates deeply with us because it mirrors exactly what we are building with our AuroraPrime platform. Here is a look at the key insights from McKinsey’s latest report and how they map to the reality of modern drug development.
The "Coworker" Effect: Unlocking 40% More Capacity
McKinsey’s research found that 75 to 85 percent of workflows in pharma contain tasks that can be automated or augmented by AI agents. The potential impact? Freeing up 25 to 40 percent of enterprise capacity.
Think about your highly paid medical writers and clinical scientists. How much of their day is spent on high-value strategy versus low-value data drudgery? McKinsey notes that agents can handle tasks previously considered too complex for automation, such as drafting regulatory documents or analyzing unstructured datasets.
By shifting the heavy lifting of drafting and data checking to AI agents, companies can redirect their human experts toward scientific innovation. This isn't about replacing people; it's about giving them a "digital sidekick" that handles the grunt work so they can focus on the science.
The Regulatory Race: From Months to Weeks
One of the most compelling insights from the report is the financial imperative of speed. McKinsey estimates that accelerating regulatory filing timelines from months to weeks could unlock roughly $180 million in net present value (NPV) for a priority asset.
The report describes a "zero-based redesign" of the submission process, where AI agents draft documents immediately after database lock. This is precisely the capability we engineered into AuroraPrime RMA.
Our platform utilizes Agentic AI to automate the creation of critical documents like Clinical Study Reports (CSRs) and Protocols. Instead of a writer staring at a blank screen and manually copying data from Tables, Listings, and Figures (TFLs), our AI agents:
Auto-populate content from upstream documents (like the Protocol and SAP).
Generate TFL summaries by reading the data directly, ensuring numbers in the text match the source tables.
Perform self-correction, identifying inconsistencies between the synopsis and the body text before a human ever reviews it.
We have seen this approach reduce CSR authoring time by 30% to 50% in pilot programs with top global pharma companies.
Escaping "Pilot Purgatory"
Here is the hard truth: McKinsey found that while nearly 80% of companies are experimenting with GenAI, 80% of them report no tangible bottom-line benefits yet. This is the "AI paradox"—the technology works, but scaling it is a nightmare.
Why? because most organizations are stuck in "pilot purgatory," running isolated experiments that don't connect to the broader enterprise ecosystem.
To break this cycle, McKinsey suggests a platform-driven approach. You cannot just have a chatbot; you need an infrastructure that integrates with your existing systems (like Veeva RIM) and adheres to strict security standards.
This is the core philosophy behind AlphaLife. We don't just offer a "writing tool"; we provide an enterprise-grade platform. AuroraPrime integrates directly with your Veeva RIM and EDC systems to create a seamless digital data flow. Our agents operate within a secure, compliance-ready environment (SOC 2, ISO 27001) that respects the rigorous demands of the pharma industry.
The Future is Agentic
McKinsey predicts that companies embracing this transformation will operate radically differently within five years. The winners will be those who stop viewing AI as a novelty and start treating it as a core component of their workforce.
At AlphaLife Sciences, we are moving beyond simple text generation to Agentic AI that can autonomously plan and execute complex writing and quality control workflows. Whether it's drafting a Patient Safety Narrative in minutes or using AI to redline a protocol amendment, the technology is ready today.
The question isn't whether AI will transform life sciences. The question is: will you be leading the change, or chasing it?
Ready to meet your new digital coworkers? Discover how AuroraPrime’s Agentic AI can transform your regulatory writing. Download the Frost & Sullivan White Paper.





