Guarding the Science: Data Isolation and Zero Trust for Enterprise-Grade AI in Life Sciences
Jan 06, 2026AI is only as trustworthy as the safeguards behind it. 🔐 In life sciences, where regulatory scrutiny is intense and data sensitivity is non-negotiable, scaling AI demands more than performance—it demands confidence, control, and rigor. At AlphaLife Sciences, we are seeing firsthand how data isolation and Zero Trust architectures are becoming the foundation for enterprise-grade AI that regulatory, medical, and clinical teams can actually rely on. 🚀 This is not theoretical security—it is practical design that enables innovation without compromising science or compliance.
The life sciences industry is at a pivotal moment. The drive for faster drug development, accelerated by advanced capabilities in areas like cell and gene therapies, means that the integration of artificial intelligence is no longer optional—it is essential. However, in an industry governed by strict regulatory standards like GxP and focused intensely on patient confidentiality, the deployment of GenAI cannot compromise security or data integrity.
For global pharmaceutical enterprises, trust isn't a feature; it’s a prerequisite. That is why AlphaLife Sciences built our flagship platform, AuroraPrime RMA (Regulatory and Medical Authoring), not just for speed—demonstrating a 90% reduction in first draft time for critical documents—but for uncompromising, enterprise-grade security.
The question for security and IT leaders is critical: How do you leverage the immense power of GenAI without exposing your most sensitive clinical data?
The answer lies in architectural isolation, transparent data handling, and an unwavering commitment to trust principles.
The Zero-Trust Mandate for Clinical Data
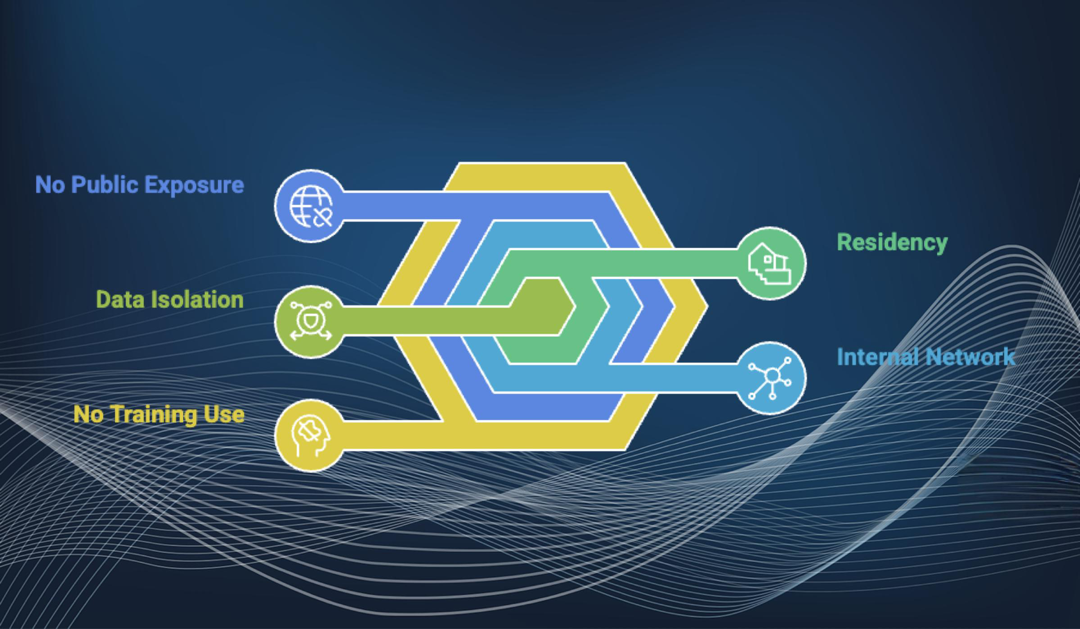
In the high-stakes world of pharmaceutical R&D, every piece of data—from individual case safety reports (ICSRs) to patient safety narratives and Clinical Study Reports (CSRs)—is classified as highly sensitive. Using off-the-shelf AI tools that send data over the public internet or use client information for model training is simply not permissible.
AuroraPrime RMA is specifically engineered to eliminate these risks by following a strict zero-trust principle. This means trust is never assumed, even within an internal network.
Our data handling principles provide comprehensive assurance:
Complete Data Isolation and Residency: The platform is meticulously designed to ensure that clinical data does not leave the client’s environment. For clients who require the strictest controls, the AuroraPrime RMA platform can be deployed entirely within your infrastructure, ensuring it operates exclusively within your internal network.
No Public Internet Exposure: It is a fundamental design requirement that there is no need to expose any service endpoints to the public internet.
Data Never Used for Training: To maintain strict data segregation and security, client data is never used for model training. This means that your proprietary knowledge remains isolated within your instance.
Protecting Data In Transit and At Rest
In the pharmaceutical ecosystem, data must be protected both when it is stored (at rest) and when it is moving between systems (in transit). AuroraPrime RMA achieves this end-to-end protection through continuous encryption:
End-to-End Data Stream Encryption: The platform supports end-to-end data stream encryption. Adhering to the zero-trust principle, this encryption extends to all data streams, including internal intranet traffic.
End-to-End Data Storage Encryption: To protect archived and current information, both the database and file storage are encrypted to protect data at rest.
Token Security: Access and service tokens are further secured using certified hardware-based encryption (HSM)—a crucial measure for anti-forgery protection.
This layered encryption ensures that your sensitive information, whether flowing through the system to generate a complex DSUR or waiting in storage, is always guarded.
Built-in Compliance and Auditing
For a GenAI solution to be effective in life sciences, it must natively support global regulatory requirements. AuroraPrime RMA adheres to a comprehensive range of global standards:
HIPAA Compliance: Our security framework is built upon the HIPAA Security Rule’s requirements, providing administrative, physical, and technical safeguards designed to protect the confidentiality, integrity, and availability of electronic protected health information (ePHI).
Global Standards: The platform adheres to internationally recognized compliance standards including FDA 21 CFR Part 11, GDPR, ISO 9001, ISO 27001, and AICPA SOC 2 Type II. Furthermore, our platform is capable of generating and performing quality control of GxP reports automatically.
Secure Access: User access is governed by Role-Based Access Control (RBAC), and the platform supports Single Sign-On (SSO) using OAuth 2 and SAML protocols. This allows seamless authentication via existing identity providers like Okta or Microsoft Entra ID.
Full Auditability: AuroraPrime RMA includes built-in logging of security actions and data changes, supporting transparency, traceability, and compliance requirements. This is crucial in the regulatory environment where accountability is key.
By ensuring strict data residency, implementing rigorous encryption protocols, and adhering to global compliance mandates, AlphaLife Sciences enables pharmaceutical companies to adopt AI with confidence. AuroraPrime RMA is not just driving efficiency; it is establishing a new standard for secure, enterprise-grade AI deployment that can support the rapid and compliant development of the next generation of life-changing treatments.





