How to Craft Effective Prompts in GenAI-Assisted Medical Writing
Oct 25, 2024In the fast-evolving world of GenAI-assisted medical writing, the key to getting accurate, high-quality outputs lies in how well you craft your prompts. This blog explores the importance of specificity and context in developing effective prompts, especially for complex medical documents like clinical study reports (CSRs). Whether you're a seasoned writer or new to GenAI tools, mastering prompt design will help you streamline workflows, improve compliance, and elevate your writing. Discover how AI can enhance your medical writing by refining drafts and automating essential tasks.

In the evolving landscape of medical writing, the integration of generative AI (GenAI) tools has revolutionized how we generate regulatory content such as Tables, Figures, and Listings (TFL) summaries. However, the effectiveness of GenAI-generated content hinges significantly on the quality of the prompts used.
When using AuroraPrime Create for clinical regulatory documents, the tool provides built-in GenAI prompts optimized for various scenarios. Additionally, it allows you to input specific prompts and examples to fine-tune outputs to meet your precise requirements.
Here, we explore best practices for crafting prompts that yield precise, compliant, and high-quality regulatory documents.
Be specific and clear
Specificity is key when writing prompts. Vague requests can lead to ambiguous results. For instance, when generating a TFL summary, instead of asking for a "summary of the Disposition of Participants table," specify key study information and highlight what you want to emphasize.
A more effective prompt might be:
“Generate a TFL summary interpreting the Disposition of Participants table. Highlight the number of participants screened, enrolled, completed the study, and reasons for discontinuation. Include a brief analysis of the retention rates and any notable trends observed in participant dropout.”
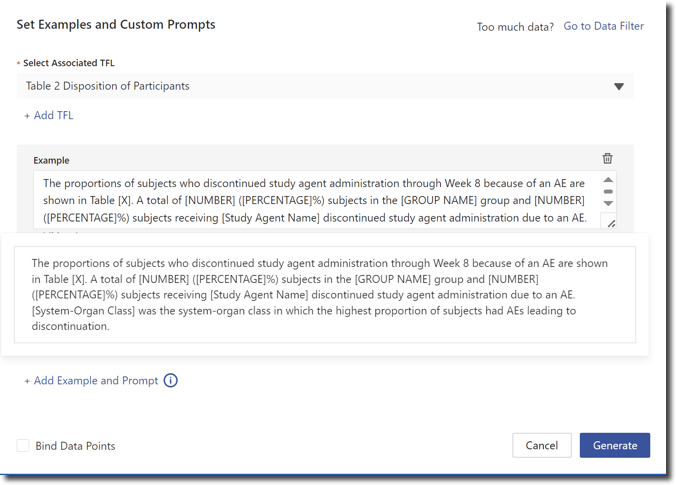
Provide examples
Whenever possible, provide examples of writing to clarify expectations and set benchmarks for the GenAI. For a TFL summary, you might share:
"The proportions of subjects who discontinued study agent administration through Week 8 because of an AE are shown in Table [X]. A total of [NUMBER] subjects in the [GROUP NAME] group and [NUMBER] subjects receiving [Study Agent Name] discontinued study agent administration due to an AE. [System-Organ Class] was the system-organ class in which the highest proportion of subjects had AEs leading to discontinuation."
Utilize contextual information
Providing context can significantly enhance the relevance of the output. Include details about the therapeutic area, study design, and any regulatory guidelines that must be followed. This is particularly important for documents like study protocols and CSRs that require adherence to specific regulatory frameworks.
Example prompt:
“Generate a TFL summary interpreting the Disposition of Participants table for a Phase II trial of Drug ABC in patients with moderate to severe asthma. ”
Review and refine
GenAI-generated content is not a final product. After generating the initial draft, thoroughly review it for accuracy, compliance, and clarity. Incorporate feedback to refine prompts for future use, improving the quality of subsequent outputs.
Example refinement:
If the GenAI’s initial draft lacked depth in the results section, adjust your prompt to emphasize data interpretation:
“Revise the text to include a detailed analysis of efficacy outcomes and their clinical significance based on statistical results.”
Encourage iterations
GenAI can be a powerful tool for iterative writing processes. Don’t hesitate to ask for multiple versions or alternative approaches. This can help uncover new insights or perspectives that may enhance your regulatory documents.
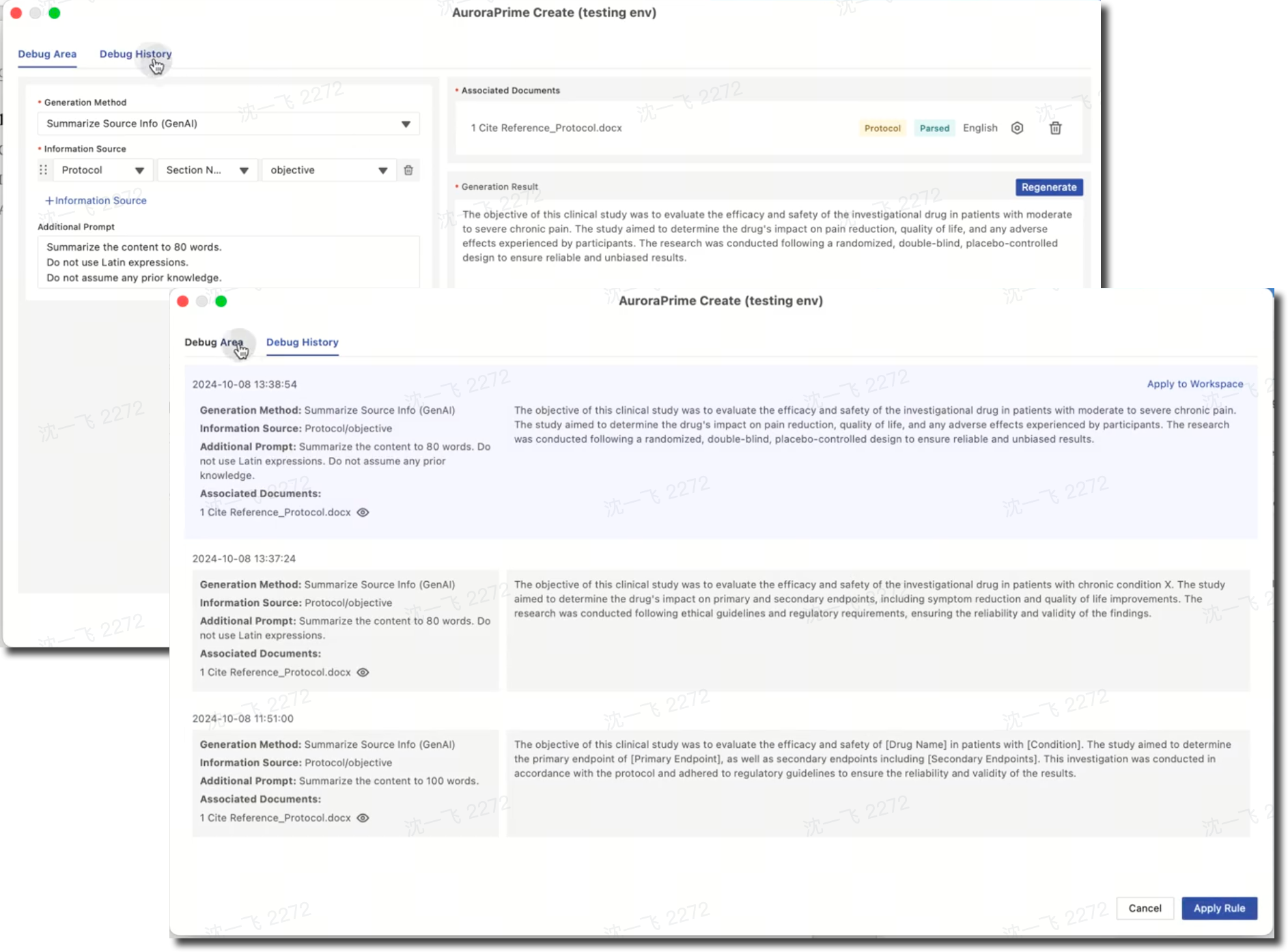
AuroraPrime Create allows you to debug GenAI generation rules when configuring document templates. By generating content using uploaded examples and additional prompts, you can review debug history and select the most satisfactory rule.
Final thoughts
GenAI-assisted medical writing holds great potential for increasing efficiency and consistency in regulatory documentation. By employing these best practices for crafting prompts, medical writers can maximize the effectiveness of GenAI tools, ensuring that CSRs, TFL summaries, and study protocols meet the high standards required for regulatory submissions. As we continue to integrate GenAI into our workflows, embracing these strategies will be essential in producing high-quality, compliant medical documents.





