The 90% Breakthrough: How AuroraPrime is Revolutionizing Clinical Study Reports
Aug 12, 2025🚀 Unlock the future of clinical documentation! With AuroraPrime, AlphaLife Sciences is delivering a breakthrough transformation—slashing first-draft time for Clinical Study Reports (CSRs) by a staggering 90%, and achieving overall CSR production time savings of 50%. This isn’t just a boost in productivity—it’s a paradigm shift for medical and regulatory writing teams. Dive in to discover how this GenAI "co-pilot" is reshaping drug development.
In the fast-paced world of drug development, every moment counts. Clinical Study Reports (CSRs) are pivotal documents, summarizing the complete efficacy and safety data of a clinical trial. Their creation is a complex, labor-intensive process, traditionally involving stringent regulatory standards, high resource demands, and intricate data management. This often leads to significant costs and extended timelines, directly impacting the speed at which life-saving treatments can reach patients.
At AlphaLife Sciences, we understand these challenges intimately. That’s why we developed AuroraPrime, our flagship Generative AI (GenAI) platform, specifically designed to transform clinical development. Our mission is to drive productivity and quality throughout the drug lifecycle through digital transformation and automation.
A Revolution in CSR Authoring: Slash Timelines by 90%
We are thrilled to share how AuroraPrime is delivering an efficiency revolution, particularly in the creation of Clinical Study Reports. With AuroraPrime, organizations can achieve a remarkable 90% reduction in first draft time for CSRs, leading to an overall time savings of 50% in CSR production. This isn't just a marginal improvement; it's a paradigm shift that fundamentally changes how medical and regulatory writing teams operate.
How AuroraPrime Drives This Unprecedented Efficiency:
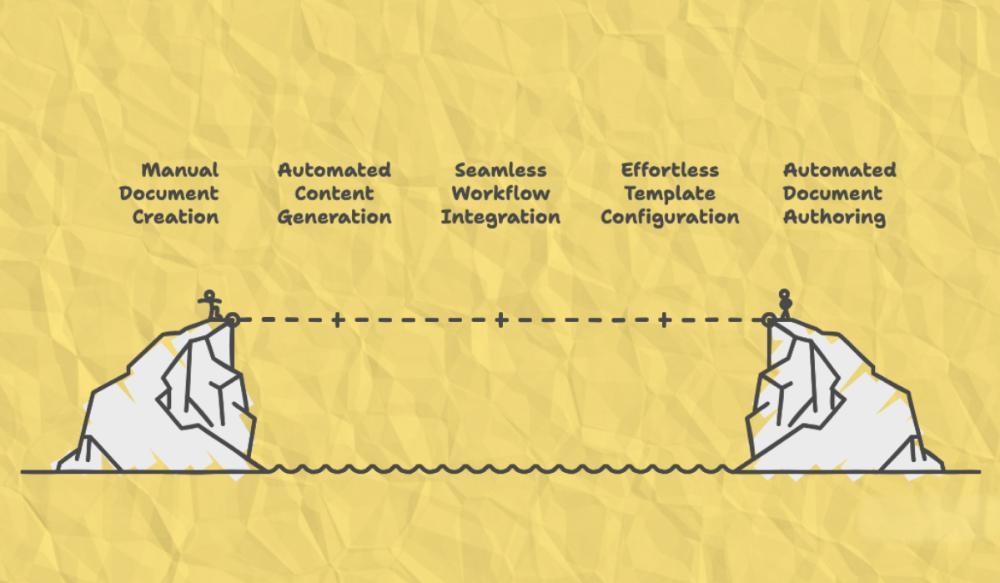
AuroraPrime acts as an intelligent "co-pilot," automating various processes from drafting and template configuration to document lifecycle management, all while ensuring content quality and compliance.
Comprehensive First Draft Automation with GenAI Quality Assurance: AuroraPrime accelerates initial drafts by automating content generation, extracting data, and structuring information. For CSRs, it reuses content from protocols and Statistical Analysis Plans (SAPs) to create data-independent sections. For data-intensive sections, it seamlessly syncs Table, Figure, and Listing (TFL) data with placeholders and automatically generates summaries, significantly reducing manual effort and ensuring regulatory alignment. The platform also incorporates a built-in GenAI quality control mechanism that compares outputs with "golden" benchmarks to assess accuracy and coverage, allowing medical writers to customize settings for specific document and regulatory needs.
Seamless Integration into Existing Workflows: Our solution is offered as a Microsoft Word add-in, integrating directly into your team’s existing workflows. This allows medical writers to work within a familiar environment while leveraging advanced automation capabilities. AuroraPrime also integrates fully with upstream and downstream systems, such as Regulatory Information Management (RIM) systems (e.g., Veeva RIM), streamlining workflows and enabling automated document authoring with minimal manual intervention.
Effortless Document Template Configuration: AuroraPrime enables detailed and flexible configuration of content references across documents. It allows users to build new templates from existing documents, automatically extracting relevant content as examples, which supports diverse therapeutic areas and document types. Its no-code, self-service configuration empowers internal teams to create and manage templates independently, accelerating setup and reducing reliance on vendor support.
Agile Development and Continuous Improvement: We follow an agile development lifecycle with a monthly release cadence, allowing us to quickly adapt to evolving requirements and continuously improve our solution based on user feedback and industry advancements. Our close partnerships with leading GenAI providers like Microsoft, Google, and Nvidia ensure we remain at the forefront of AI innovation, rapidly adopting the latest models like GPT-4o for enhanced accuracy and speed.
Beyond CSRs: A Broader Impact on Drug Development
The efficiency gains from AuroraPrime extend beyond just CSRs, impacting the entire clinical development process. According to McKinsey analyses, Generative AI can generate $13–$25 billion annually in clinical development efficiency gains, potentially cutting clinical trial costs by up to 50% and trimming trial timelines by over a year.
For medical writers, this means:
Efficiency Gains: Freeing up valuable time for more complex, strategic tasks.
Reduced Manual Tasks: Automating repetitive and tedious work, minimizing human error.
Regulatory Compliance: Ensuring content aligns with stringent regulatory standards, enhancing precision and quality.
AlphaLife Sciences has a proven track record, chosen by 5 of the top 10 global pharma companies and leading CROs, and is backed by prestigious programs such as Johnson & Johnson Innovation JLABS and Microsoft's Pegasus Program. Our solution consistently ranks as a top choice in global RFP processes due to its effectiveness and reliability.
Join the Efficiency Revolution
The transformative potential of GenAI in clinical development is undeniable. AuroraPrime embodies this potential, offering end-to-end automation in medical writing that enhances quality, efficiency, and compliance across critical documentation processes.
We invite you to connect with AlphaLife Sciences to explore how AuroraPrime can deliver transformative gains in your clinical documentation workflows and accelerate the delivery of life-saving treatments worldwide.





