The Enterprise Leap: Architecting the Global Scale of GenAI in Pharmaceutical R&D
Dec 04, 2025🚀 The life sciences industry is standing at a major inflection point — and the organizations that scale GenAI effectively today will shape how R&D operates tomorrow. At AlphaLife Sciences, we’re seeing firsthand how global pharma teams can move from individual pilots to true enterprise-wide impact, transforming regulatory, medical, and clinical workflows with speed, consistency, and trust.From unified data foundations to AI-enabled process orchestration, the path to scalable GenAI isn’t theoretical anymore — it’s happening in real programs, across real teams, delivering measurable results. If you’re exploring how to architect GenAI for global R&D operations, this perspective will spark ideas on what’s possible next.
The pharmaceutical industry, facing relentless pressure for speed, quality, and rigorous compliance, is undergoing a profound digital transformation. As we look ahead, the global clinical landscape is continually evolving, driven by emerging therapies and technological advancements. Complex areas like oncology, cell and gene therapies require innovation that moves beyond traditional, manual workflows.
Generative AI (GenAI) offers the essential pathway to achieve this efficiency, but the journey from a single Proof-of-Concept (POC) to scaling GenAI across a global enterprise is complex, demanding robust technology, proven reliability, and a clear implementation roadmap.
At AlphaLife Sciences, we specialize in making this leap successful. Our flagship AuroraPrime RMA (Regulatory and Medical Authoring) platform, and the GenAI solutions built upon it, are engineered not just for pilots, but for full-scale production, backed by a demonstrated track record with the world’s largest pharmaceutical organizations.
Establishing Trust: The Rigor of Pilot Validation
Global pharmaceutical companies operate under the strictest regulatory standards, meaning any new technology must undergo rigorous vetting. The successful adoption of GenAI starts with meticulous validation, moving beyond theoretical potential to proven, compliant performance.
AuroraPrime is a platform proven in global pharmaceutical deployments and a trusted regulatory and medical authoring solution. Our commitment to demonstrated performance and reliability is evident: we are currently trusted by over half of the Top-20 Global Pharma, and 5 out of the top 10 global pharma companies have integrated AuroraPrime into their critical workflows.
This trust is earned through a structured validation process:
Global Market Scans and RFPs: We consistently rank as a top choice in global RFP processes due to the effectiveness and reliability of our AI-powered solutions.
Targeted Proof-of-Concept (POC) Programs: We successfully conduct GenAI pilot programs focused on high-value clinical documents. These programs demonstrably build confidence in AuroraPrime’s capabilities and pave the way for smooth transitions to full production.
For instance, we demonstrated the rapid generation of documents like Clinical Study Reports (CSRs) and Development Safety Update Reports (DSURs). This has translated into real-world efficiency gains, showing a 90% reduction in first draft time and a 45% to 50% overall time reduction for CSRs in real-world deployments.
The Implementation Roadmap: From POC to Production
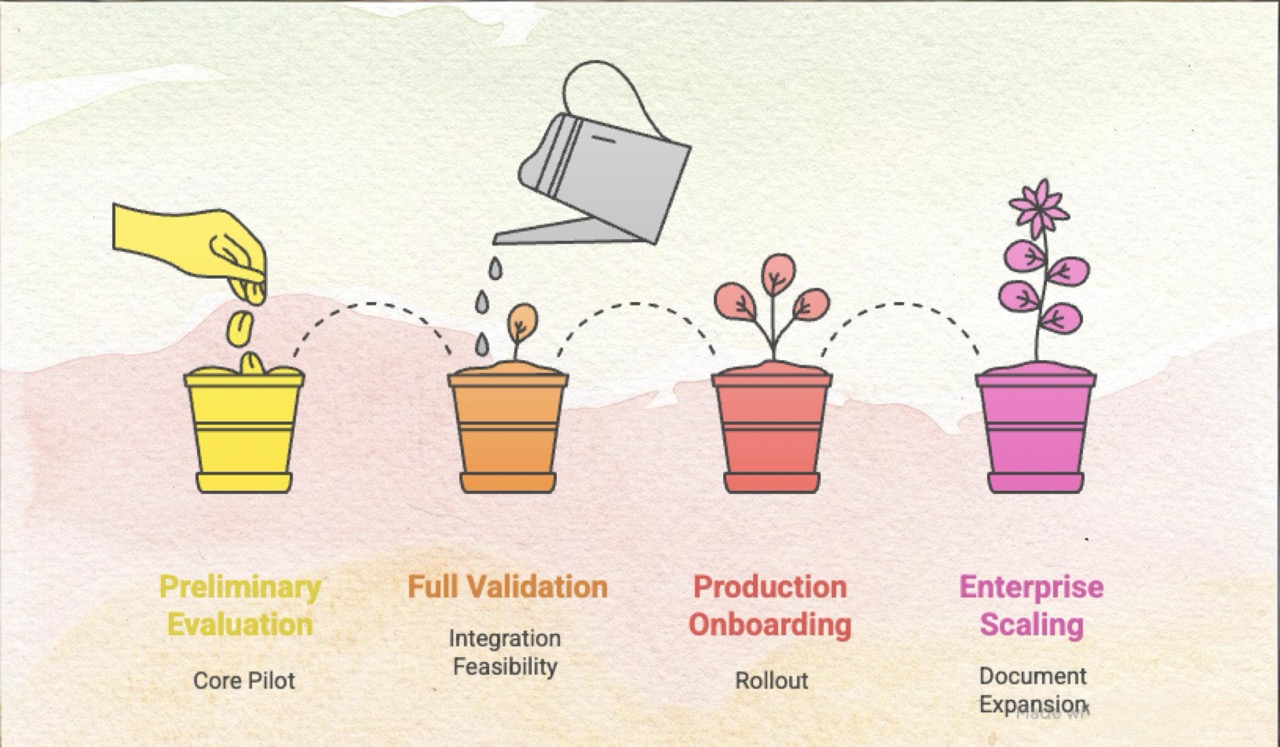
The transition from a confined pilot to enterprise-wide utilization requires a staged, agile implementation plan that minimizes risk while maximizing time-to-value. Our roadmap typically unfolds in four key stages, ensuring both rigorous validation and seamless expansion:
Stage 1: Preliminary Evaluation (The Core Pilot)
This initial stage allows the client’s medical writers to evaluate AuroraPrime’s core capabilities, often using the client’s own templates and representative trial data within a secure SaaS environment (e.g., AlphaLife Azure). The goal here is to assess usability, content quality, and alignment with existing authoring workflows.
Stage 2: Full Validation and Integration Feasibility
The crucial second phase involves a containerized deployment of AuroraPrime RMA directly within the client’s scoped infrastructure (e.g., Gilead’s AWS environment). This stage validates business workflows, integration feasibility (e.g., connecting via REST APIs to systems like Veeva Vault or internal DnA platforms), and user experience using real data and templates.
Stage 3: Production Onboarding and Rollout
Once validation is complete, the solution moves to a full production deployment, typically starting with early adopters across specific therapeutic areas. This stage involves User Acceptance Testing (UAT), full production rollout, and essential change management services.
Stage 4: Enterprise Scaling and Document Expansion
The final and most strategic stage involves sustaining the solution and expanding support across the entire enterprise to support a broader range of document types.
A Success Story in Scaling: Johnson & Johnson
A prime example of this success is our ongoing collaboration with Johnson & Johnson (J&J). After a rigorous global market scan, RFP process, and proof of concept (POC), AlphaLife Sciences was selected as the sole vendor to support the J&J medical writing team’s AI-assisted authoring tool. J&J has since expanded the partnership to support a broader range of document types at the enterprise level and global scale, highlighting AuroraPrime’s ability to meet complex, high-stakes requirements.
Engineered for Global Enterprise Scale
Scaling GenAI across a global pharmaceutical enterprise demands an architecture that is flexible, highly secure, and deeply integrated with existing R&D ecosystems. AuroraPrime RMA is purpose-built to meet these demands.
Flexibility and Agnosticism
For global adoption and future-proofing, technological flexibility is paramount:
Cloud-Agnostic Architecture: AuroraPrime is built on a Kubernetes-based microservices architecture, enabling modular deployment across diverse environments and full support for multi-cloud deployment (e.g., client AWS EKS or Azure environments).
LLM Agnosticism: The platform’s design allows clients the flexibility to seamlessly switch between different Large Language Models (LLMs) (such as GPT-4.1, Gemini, Claude, and Llama) or integrate with client-hosted AI models. This ensures sustained optimal performance regardless of future AI advancements.
Deep System Integration
Enterprise-level GenAI cannot exist in a silo; it must operate seamlessly within the complex document ecosystem. AuroraPrime RMA achieves this through:
Native Microsoft Word Integration: The add-in operates seamlessly within Microsoft Word 365, transforming a familiar tool into an AI-powered environment, allowing writers to leverage collaboration features without switching applications.
Veeva RIM Connectivity: AuroraPrime offers API-driven integration with Veeva Vault RIM, ensuring deep connectivity for efficient data extraction and streamlined file management. This addresses the core challenge of fragmented workflows.
Quality and Compliance at Scale
Scaling cannot compromise quality. Our platform is designed with a comprehensive AI Quality Control Framework and a rigorous approach to compliance:
Human-in-the-Loop (HITL): This model ensures medical writers remain in full control of the end-to-end process. Writers provide feedback (e.g., "thumbs up" or "thumbs down") on AI-generated content, which is retained and actively used to continuously refine the AI's performance.
Traceability and Auditability: The platform meticulously logs all AI-generated content and user feedback within the AI Tasks pane, creating a clear audit trail for compliance with standards like GxP.
AlphaLife Sciences is dedicated to driving productivity and quality throughout the drug lifecycle by transforming medical and regulatory writing. By providing a scalable, auditable, and production-proven solution, we empower global pharma organizations to confidently move GenAI From Pilot to Production and accelerate life-changing treatments to market.





