The Evolution of Medical Writing—From Manual Processes to AI-Driven Efficiency
Jun 16, 2025📜✍️ From Pen & Paper to Prompt Engineering 🤖⚙️Medical writing has come a long way—from labor-intensive manual processes to lightning-fast, AI-powered precision. But what does this evolution mean for the future of regulatory content, clinical documentation, and medical affairs? Dive into how AI is not just a tool but a game-changer in life sciences writing. 🧠💡
In the dynamic landscape of life sciences, medical writing serves as the vital conduit connecting complex clinical data to clear, compliant documentation. For decades, this critical function relied heavily on manual processes – the painstaking task of extracting, verifying, and integrating data into essential documents like Clinical Study Reports (CSRs), Protocols, and Safety Narratives.
This traditional approach, while foundational, presented significant challenges. Medical writers often faced strict regulatory compliance requirements, demanding high resource allocation and complex data management. Frequent data updates and multi-team coordination added layers of complexity and increased the risk of errors. Manual, labor-intensive processes could lead to inefficiencies, delays, and ultimately, increased costs in the high-stakes pharmaceutical industry.
The field has undergone a significant transformation, moving from ancient manuscripts to digital documentation. Now, we are witnessing another pivotal shift driven by Artificial Intelligence (AI). The question is no longer if AI will impact medical writing, but how it will empower writers and reshape workflows.
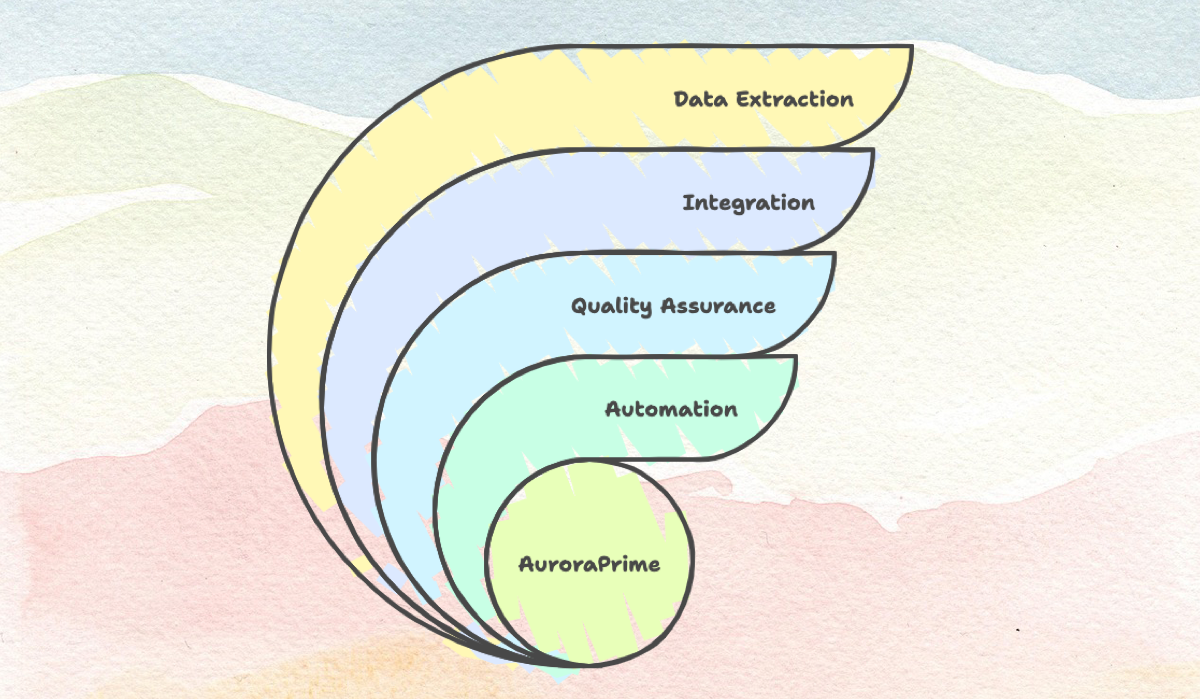
At AlphaLife Sciences, we are at the forefront of this evolution, recognized as a global leader in Generative AI-driven clinical development. Our flagship AuroraPrime platform isn't just an AI tool; it's a unified, GenAI-powered solution designed to fundamentally integrate medical authoring and data management, streamlining workflows and boosting clinical lifecycle efficiency. Offered as a convenient Microsoft Word add-in, AuroraPrime allows medical writers to harness cutting-edge AI within their familiar environment.
Ensuring data adheres to critical industry standards is paramount for regulatory success. Our commitment to data standards is solidified by AlphaLife Sciences' status as a Gold Member of CDISC. This strategic membership allows us to directly integrate global data standards, such as SEND, SDTM, and ADaM, into the AuroraPrime platform.
Why does our CDISC Gold Membership matter for your documentation? It means AuroraPrime is built to enable seamless compliance by auto-generating CDISC-aligned clinical trial documents. This drastically reduces manual errors and accelerates FDA/EMA submissions. Furthermore, it helps enhance cross-stakeholder alignment with standardized data inputs, leading to less rework for sponsors and CROs. As a CDISC Gold Member, AlphaLife Sciences is also actively positioned to help shape the future of clinical data standards, ensuring AuroraPrime remains a leading, compliance-driven platform for enterprises.
AuroraPrime leverages GenAI to address the historical pain points of medical writing and unlock new levels of efficiency and quality. Here’s how AuroraPrime is driving the evolution of medical writing:
Accelerated First Draft Generation: AuroraPrime automates the creation of initial drafts for crucial documents. For CSRs, it reuses content from Protocols and Statistical Analysis Plans (SAPs) to auto-populate data-independent sections. Protocols can be drafted based on a synopsis or even study ideas, automatically generating abbreviations and the Schedule of Events (SoA). Patient Safety Narratives can be auto-generated in batches within minutes by directly integrating data from EDC and PV systems.
Automated TFL Management and Synchronization: Handling Tables, Figures, and Listings (TFLs) is often manual and error-prone. AuroraPrime inserts TFL placeholders and automatically generates TFL summaries, keeping them synchronized with the source TFL data. This eliminates tedious manual updates and ensures consistency.
Intelligent Content Reuse and Knowledge Integration: The platform builds upon a comprehensive Knowledge Base that can include previous trial documents, data, company content assets, and integrates external literature sources. This allows for intelligent content reuse and leverages Retrieval-Augmented Generation (RAG) to ensure accuracy and relevance.
Built-in Quality Assurance: To ensure accuracy and compliance, AuroraPrime includes built-in GenAI Quality Assurance. It can compare generated content against "golden benchmarks" derived from exemplary historical documents, evaluating quality and coverage. Automated checks help ensure accuracy and adherence to standards and can auto-detect inconsistencies.
Flexible, No-Code Template Management: Creating and managing document templates is simplified with AuroraPrime's flexible, no-code configuration. Templates can be easily set up for different therapeutic areas or document types and built from existing documents.
Seamless Ecosystem Integration: AuroraPrime integrates with essential systems beyond just Word. It connects with RIM systems, Microsoft 365 (for review workflows), EDC and PV systems, CTMS, eTMF, RTSM, DCT, and literature databases. This streamlines workflows and ensures data flows efficiently.
Extracting Value from Unstructured Data: For documents like ICSRs, AuroraPrime can extract unstructured content from diverse sources (literature, forms, reports, etc.) and structure it for use. It can also extract key data points for documents like Lay Summaries.
By automating repetitive tasks and seamlessly integrating data and knowledge, AuroraPrime allows medical writers to reduce manual effort and dedicate more time to high-value activities such as complex analysis, data interpretation, strategic thinking, and crafting the nuanced narratives that clinical documents require. This leads to significant time savings (30-50% overall for CSRs, 45% for Protocols, 70% for Safety Narratives, validated by top pharma and CROs), cost reduction, improved quality and consistency, and accelerated timelines, contributing to faster time to market for life-saving treatments.
AlphaLife Sciences is trusted by top global pharmaceutical companies and CROs, validating the platform's ability to meet stringent industry standards. We are supported by strategic partnerships with industry leaders like Microsoft, NVIDIA, Veeva, and Google, as well as prestigious programs such as Johnson & Johnson Innovation JLABS.
The evolution of medical writing is not about replacing human expertise with machines. It's about a powerful partnership between skilled medical writers and advanced AI tools like AuroraPrime. It's about moving beyond manual processes and embracing a future where technology enhances human capabilities, ensuring documents are not only compliant and accurate but also deeply informed by the data they represent. This is the future of clinical documentation, and it's here today.





