Unlocking Regulatory Velocity: How AuroraPrime's AI Transforms Life Sciences Documentation
Sep 29, 2025🚀 Unleashing AI in Life Sciences: Regulatory Velocity Redefined 🌐What if the enormous documentation hurdle in drug development could be turned into your competitive edge? AuroraPrime’s generative AI is doing just that — compressing weeks of regulatory drafting into mere minutes, infusing compliance intelligence, and giving medical writers back their time. Imagine accelerating clinical timelines and raising the bar on quality. That’s not science fiction — that’s here.
In the dynamic world of life sciences, bringing a new drug or therapy to patients is a monumental undertaking. It’s a journey paved with rigorous research, complex clinical trials, and, crucially, an immense volume of regulatory documentation. This documentation, meticulously prepared and submitted to regulatory authorities like the FDA, is the bridge between scientific breakthrough and patient access. But what if this critical bridge could be built faster, with greater precision, and unwavering compliance? At AlphaLife Sciences, we believe it can, and our flagship AuroraPrime Generative AI platform is making it a reality.
The Documentation Hurdle: A Bottleneck to Breakthroughs
Traditionally, the process of authoring regulatory documents has been a significant bottleneck in drug development. It’s often characterized by manual, time-consuming processes, fragmented data sources, and a constant struggle for consistency and accuracy across a multitude of complex documents. Medical writers spend countless hours on tasks like incorporating Tables, Figures, and Listings (TFLs) and generating summaries, which are essential yet labor-intensive. This inefficiency not only delays timelines for regulatory submissions but also diverts valuable human resources from more strategic, innovative work.
Introducing AuroraPrime: Revolutionizing Medical Content Authoring with AI
The AuroraPrime Platform is an advanced, AI-powered solution specifically designed to transform medical and regulatory content authoring. It delivers agentic solutions that enhance the automated creation of a wide range of regulatory documents across all phases of R&D, including pre-clinical, clinical, regulatory, and safety areas. AuroraPrime is built on a modular AI and Large Language Model (LLM) framework, allowing flexible integration with existing enterprise systems to streamline the entire product development lifecycle.
Accelerating Documentation: Speed and Efficiency Unleashed
One of the most compelling impacts of AuroraPrime is the dramatic reduction in document creation time. For critical documents like Clinical Study Reports (CSRs), AuroraPrime boasts a 90% reduction in first draft time and a 50% overall time reduction. Similarly, Protocols see a 90% first draft time reduction and 50% overall time savings, while Safety Narratives benefit from a remarkable 95% reduction in first draft time and 70% overall time savings. This means processes that traditionally take weeks can now be accomplished within minutes.
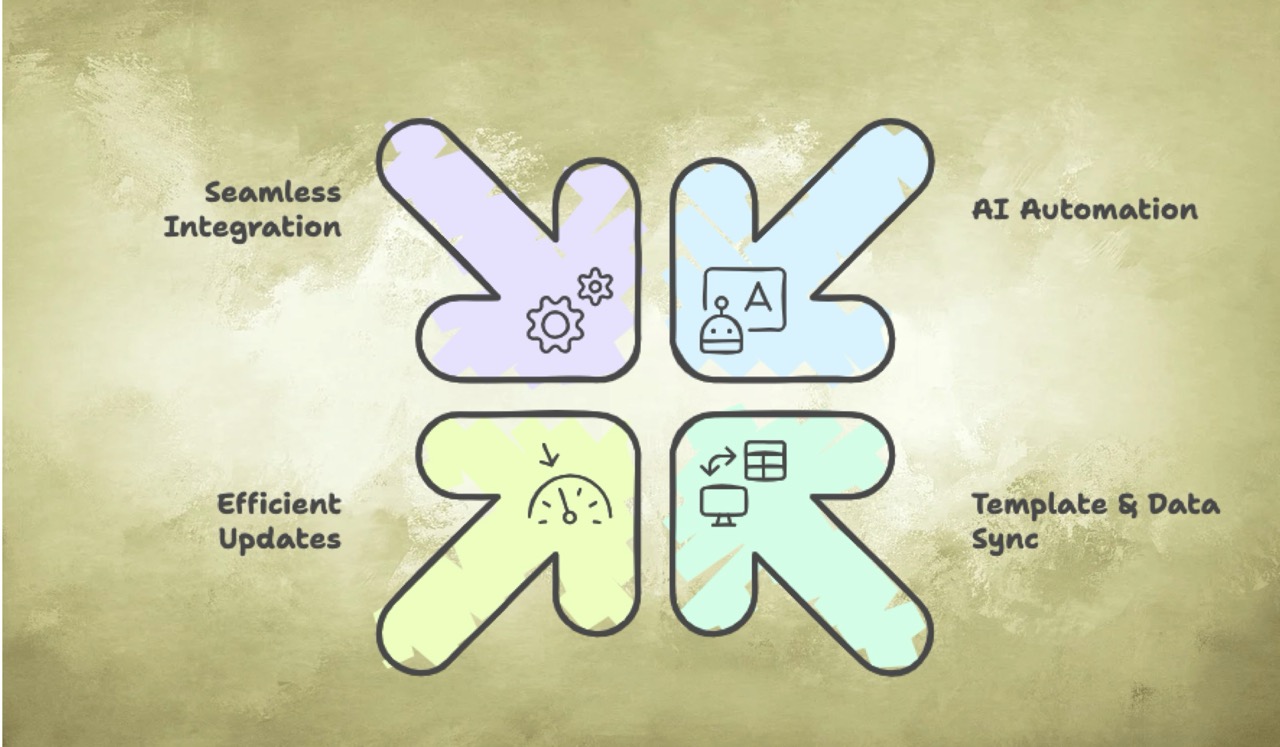
How does AuroraPrime achieve this?
AI Automation speeds up writing by generating accurate medical documents swiftly with minimal manual effort. It can auto-generate initial drafts using AI-enabled templates, and even automatically generate summaries of TFLs.
Template & Data Sync uses enterprise templates and synchronizes TFL data to streamline setup and drafting.
Efficient Updates enable one-click batch updates, ensuring documents remain current and compliant. AuroraPrime can automate batch content generation and batch TFL incorporation.
Seamless Integration allows the platform to connect smoothly with existing systems like Microsoft Word 365, Veeva RIM, EDC platforms, and Pharmacovigilance (PV) systems, unifying content and maintaining consistency across platforms. The AuroraPrime RMA add-in operates directly within Microsoft Word, allowing medical writers to leverage AI features without switching applications.
Elevating Quality and Ensuring Compliance
Beyond speed, AuroraPrime significantly enhances the quality and compliance of regulatory documentation. Regulatory writing demands absolute precision and adherence to strict guidelines.
AuroraPrime ensures this through:
Regulatory Compliance checks documents for accuracy and compliance, aligning with global regulatory standards such as FDA 21 CFR Part 11, HIPAA, GDPR, ISO 9001, ISO 27001, and AICPA SOC 2 Type II. It embeds regulatory intelligence directly into the writing process, ensuring every draft aligns with current guidelines, industry standards, and company-specific rules.
Built-in GenAI QC ensures precision by benchmarking against "golden" standards and performing automated integrity and consistency checks.
Human-in-the-Loop AI Interaction means that while AI automates, human expertise remains in control. Medical writers can interact with an AI chat assistant, provide feedback on AI-generated content (e.g., TFL summaries, polished text, translations), and this feedback is used to continuously improve AI performance and content accuracy.
Data Traceability is robust, with all AI-generated content traceable via the AI Tasks pane, where user feedback and rationale for edits are recorded, creating a clear audit trail for review and validation.
The Direct Path to Faster Regulatory Approvals
The efficient and high-quality documentation produced by AuroraPrime directly impacts the regulatory approval process. By significantly speeding up document creation and ensuring compliance from the outset, AuroraPrime helps pharmaceutical companies achieve quicker regulatory submissions and faster market access. The FDA recognizes the increased use of AI throughout the drug product lifecycle and is committed to a risk-based regulatory framework that promotes innovation while ensuring drug safety and effectiveness. AuroraPrime's robust AI quality governance aligns with this vision.
Ultimately, this means:
Accelerated Market Entry: Reducing the time spent on documentation shortens the overall drug development lifecycle, bringing life-saving treatments to patients sooner.
Enhanced Productivity: R&D leaders are freed from routine documentation tasks, allowing them to focus on core innovation and scientific challenges.
Reduced Compliance Risk: Automated checks and built-in regulatory intelligence minimize errors, reducing the likelihood of costly delays or rejections during review.
Proven Success with Industry Leaders
AlphaLife Sciences isn't just conceptualizing the future; we're building it. AuroraPrime is a platform proven in global pharmaceutical deployments, trusted by a growing number of global pharmaceutical companies and leading Contract Research Organizations (CROs). Five out of the top 10 global pharma companies have integrated AuroraPrime into their critical workflows. One top-5 global pharmaceutical company stated, “AlphaLife Sciences sets the industry standard. Your innovative solutions are transformative—enhancing efficiency and quality across our operations”. Another top-10 global pharmaceutical company confirmed a 30% time-saving in their Medical Writing Team through a pilot using two full historical CSRs. These real-world deployments demonstrate measurable time savings and significant efficiency gains across various document types.
Partnering for a Faster, Safer Future
The journey from scientific discovery to approved therapy is complex, but with AuroraPrime, the documentation phase no longer needs to be a hurdle. By leveraging AI to empower medical writers with unprecedented speed, quality, and compliance, AlphaLife Sciences is accelerating regulatory approval and driving transformative outcomes for the entire life sciences industry. We're not just selling a product; we're offering a partnership to build the future of drug development, together.





