Unlocking Safer Medicines: How Generative AI is Revolutionizing Pharmacovigilance
May 19, 2025🔍💊 What if AI could predict adverse drug reactions before they happen?Generative AI is not just transforming the way we develop medicines—it's reshaping how we keep patients safe.From automated signal detection to intelligent literature screening, GenAI is streamlining pharmacovigilance workflows, cutting down manual review time, and uncovering hidden safety insights faster than ever before.Discover how this powerful tech is ushering in a new era of drug safety 👉
In the dynamic world of pharmaceutical development, ensuring the safety of life-saving treatments is paramount. Traditional pharmacovigilance systems face increasing challenges in processing the vast amounts of data and unstructured information crucial for identifying and managing drug-related risks. But a new era has dawned, powered by Generative AI (GenAI), and at AlphaLife Sciences, we are at the forefront of this revolution with our flagship AuroraPrime platform.
AlphaLife Sciences is a global leader in Generative AI-driven clinical development, and our AuroraPrime platform is specifically designed to optimize medical and regulatory documentation, including within pharmacovigilance. Trusted by 4 of the top 10 global pharma companies and leading CROs, and backed by prestigious programs such as Johnson & Johnson Innovation JLABS, Microsoft's Pegasus Program, and Google for Startups, we are accelerating the delivery of safer medicines to patients worldwide.
The emergence of Large Language Models (LLMs) presents revolutionary opportunities for pharmacovigilance. Traditional systems often struggle with processing massive datasets and understanding natural language text. LLMs, with their powerful natural language processing, knowledge learning, and generation capabilities, effectively complement and even surpass the limitations of these traditional systems in several key areas.
Here’s how GenAI, particularly through platforms like AuroraPrime, is transforming pharmacovigilance:
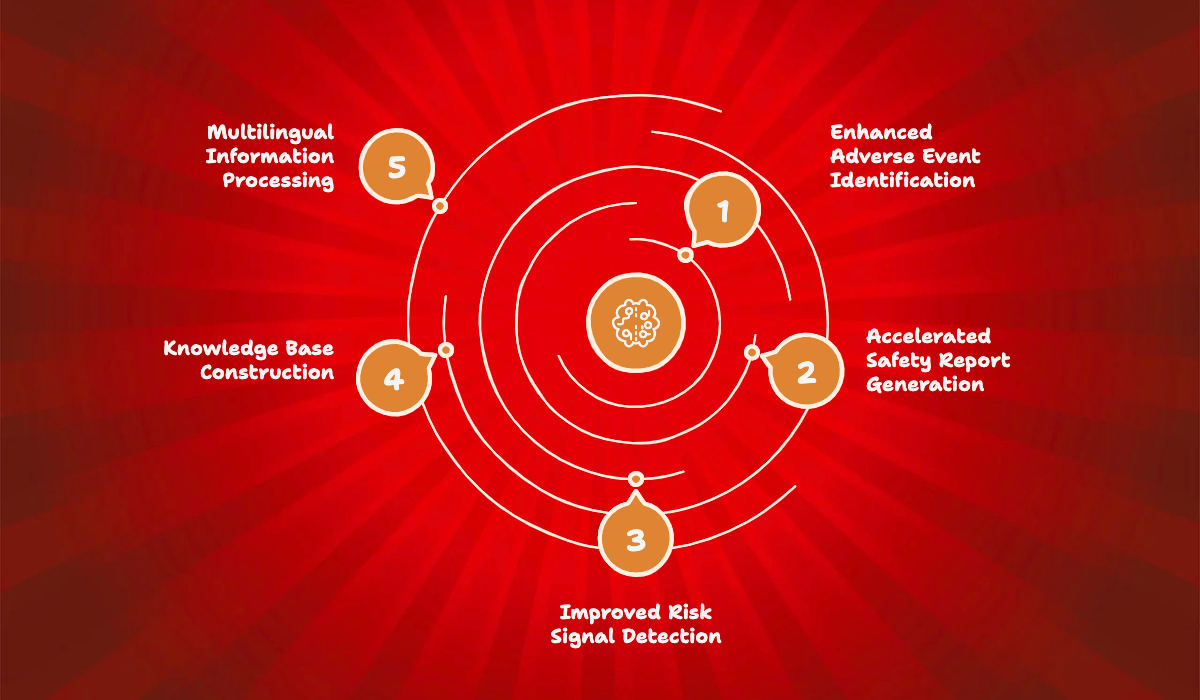
Enhanced Adverse Event Information Identification and Extraction: GenAI can more accurately identify and extract critical adverse event information from unstructured data sources like case reports, medical literature, and social media, improving efficiency and comprehensiveness.
Accelerated and Automated Safety Report Generation: Our AuroraPrime RMA platform can automate the generation of crucial safety documents such as Patient Safety Narratives, Individual Case Safety Reports (ICSRs), and Development Safety Update Reports (DSURs) / Periodic Safety Update Reports (PSURs). This significantly reduces report writing time, enhancing efficiency and quality. The AuroraPrime Medical Narratives module within the platform facilitates the efficient generation of comprehensive reports of individual participant experiences using generative AI. The workflow involves Data Source Management to import clinical trial datasets, Data Mapping to link data paths to medical narrative templates, configuring AI Augmentation Strategies with rules and prompts, and finally generating and downloading Safety Narratives.
Improved Risk Signal Detection and Analysis: GenAI can analyze vast amounts of safety data to identify potential drug safety signals and assist in risk assessment and prediction, enabling timelier detection and management of drug risks.
Facilitation of Knowledge Base Construction and Utilization: GenAI aids in building and maintaining pharmacovigilance knowledge bases by integrating medical literature, guidelines, historical data, and more, providing comprehensive information support for safety surveillance activities.
Multilingual Information Processing and Analysis: With robust translation capabilities, GenAI can process and analyze drug safety information from various global regions, breaking down language barriers. Our AuroraPrime platform integrates GenAI translation solutions.
AuroraPrime: Your Partner in Next-Generation Pharmacovigilance
At AlphaLife Sciences, our AuroraPrime platform and RMA products are designed and developed based on the understanding that GenAI serves as a powerful complement and upgrade to traditional pharmacovigilance systems. Our platform utilizes advanced GenAI technologies, including Retrieval-Augmented Generation (RAG) and Microsoft’s PIKE-RAG, combined with our deep life sciences domain knowledge and stringent quality control systems, to provide end-to-end automated pharmacovigilance documentation solutions for pharmaceutical companies and CROs. Our RMA plugin seamlessly integrates with Microsoft Word 365, allowing medical writers to work efficiently in a familiar environment.
Looking Ahead: The Future of Pharmacovigilance with AI
We believe the future holds even more transformative potential. Within the next 3-5 years, we anticipate disruptive innovations driven by next-generation GenAI technologies, leading to more intelligent and autonomous pharmacovigilance workflows. This includes:
Deeper Automation and Intelligent Report Generation: Moving beyond template-based generation to more autonomous integration and analysis of data from sources like EDC (Electronic Data Capture) and PV (Pharmacovigilance) databases to generate insightful report drafts, leveraging technologies like Agentic AI and our use of Microsoft’s PIKE-RAG for enhanced reasoning.
Multimodal AI in Signal Detection and Risk Prediction: Integrating and analyzing diverse data types such as imaging data, genomic data, wearable device data, and even social media information for a more comprehensive understanding of drug safety profiles through Multimodal Data Ingestion capabilities.
AI-Driven Quality Control and Consistency Assurance: Automating the checking of report completeness, accuracy, logic, and consistency against internal knowledge bases and external regulatory standards, building upon our Automated Quality Check feature with continuous optimization based on “golden datasets” and user feedback.
Organizational Changes and Talent Skill Transformation: Fostering greater cross-departmental collaboration and the emergence of new roles like “Content Librarian” and “Reviewer” focused on AI model management and quality assessment.
AlphaLife Sciences is committed to being a leader in the GenAI-driven clinical development space. Our AuroraPrime platform and RMA product have already been validated by numerous leading global pharmaceutical companies and CROs. We are confident that through continuous technological innovation and close collaboration with the industry, LLM technology will play an increasingly vital role in pharmacovigilance, making a greater contribution to ensuring patient safety worldwide.





