Unlocking Velocity: How AuroraPrime's AI Transforms the Toughest Clinical Documentation Challenges
Aug 25, 2025🚀 Unlocking Velocity in Life Sciences! 🧬Clinical documentation has long been one of the toughest hurdles in drug development. But what if AI could change the game?Discover how AuroraPrime's AI-driven approach is transforming complex regulatory writing into a faster, smarter, and more accurate process—unlocking new levels of efficiency for the entire industry.
In the relentless pursuit of life-saving therapies, every phase of drug development demands precision, speed, and unwavering compliance. Nowhere is this more apparent than in clinical documentation, the meticulous record-keeping that underpins every scientific breakthrough and regulatory submission. Yet, for too long, this critical process has been riddled with bottlenecks, turning what should be a clear pathway into a complex maze.
At AlphaLife Sciences, we understand these challenges intimately. That's why our flagship AuroraPrime Generative AI platform and its suite of solutions are purpose-built to dismantle these barriers, transforming the landscape of medical and regulatory writing for the life sciences industry.
Let's shine a light on the common pain points that have historically plagued clinical documentation, and then reveal how AuroraPrime is providing effective, AI-powered solutions.
The Hidden Hurdles of Traditional Clinical Documentation
Medical and regulatory writing teams face a demanding array of tasks, often under intense pressure. Here are some of the most persistent pain points:
Manual, Time-Consuming Processes: The creation of complex regulatory documents like Clinical Study Reports (CSRs) and Protocols traditionally involves vast manual effort, making it highly time-intensive and resource-heavy. Generating initial drafts alone can take weeks.
Data Fragmentation and Inconsistencies: Clinical trials generate enormous volumes of diverse data, including complex tabular data like Tables, Figures, and Listings (TFLs) and Schedules of Assessments (SOAs) from various sources such as RTF and Excel files. Integrating these inconsistent data sources and ensuring their accuracy across documents is a major hurdle that can lead to errors and hamper overall accuracy.
Delayed Timelines to Market: Inefficient workflows and the sheer manual labor involved in documentation directly contribute to delayed timelines for regulatory submissions, slowing down crucial decision-making and innovation. This impacts the speed at which new therapies can reach patients.
Ensuring Unwavering Quality, Accuracy, and Compliance: Regulatory writing demands absolute precision and adherence to strict global standards like FDA 21 CFR Part 11, HIPAA, GDPR, ISO 9001, ISO 27001, and AICPA SOC 2 Type II. Manual processes are prone to inconsistencies and errors, increasing compliance risks and requiring extensive quality control.
Limited Scalability and Adaptability: Organizations struggle to efficiently support a diverse range of document types and adapt to evolving regulatory requirements without significant redevelopment efforts. Scaling documentation for an entire enterprise presents a major challenge.
Complex System Integration: Seamlessly integrating documentation platforms with existing enterprise systems, such as Electronic Data Capture (EDC) platforms, Pharmacovigilance (PV) systems, Regulatory Information Management (RIM) systems (like Veeva), and common authoring tools like Microsoft Word, is often technically challenging.
AuroraPrime's Transformative Solutions
AuroraPrime, our generative AI-powered platform, provides agentic solutions specifically designed to tackle these pain points, revolutionizing content authoring and documentation across the life sciences R&D lifecycle.
Here’s how AuroraPrime transforms these challenges into opportunities:
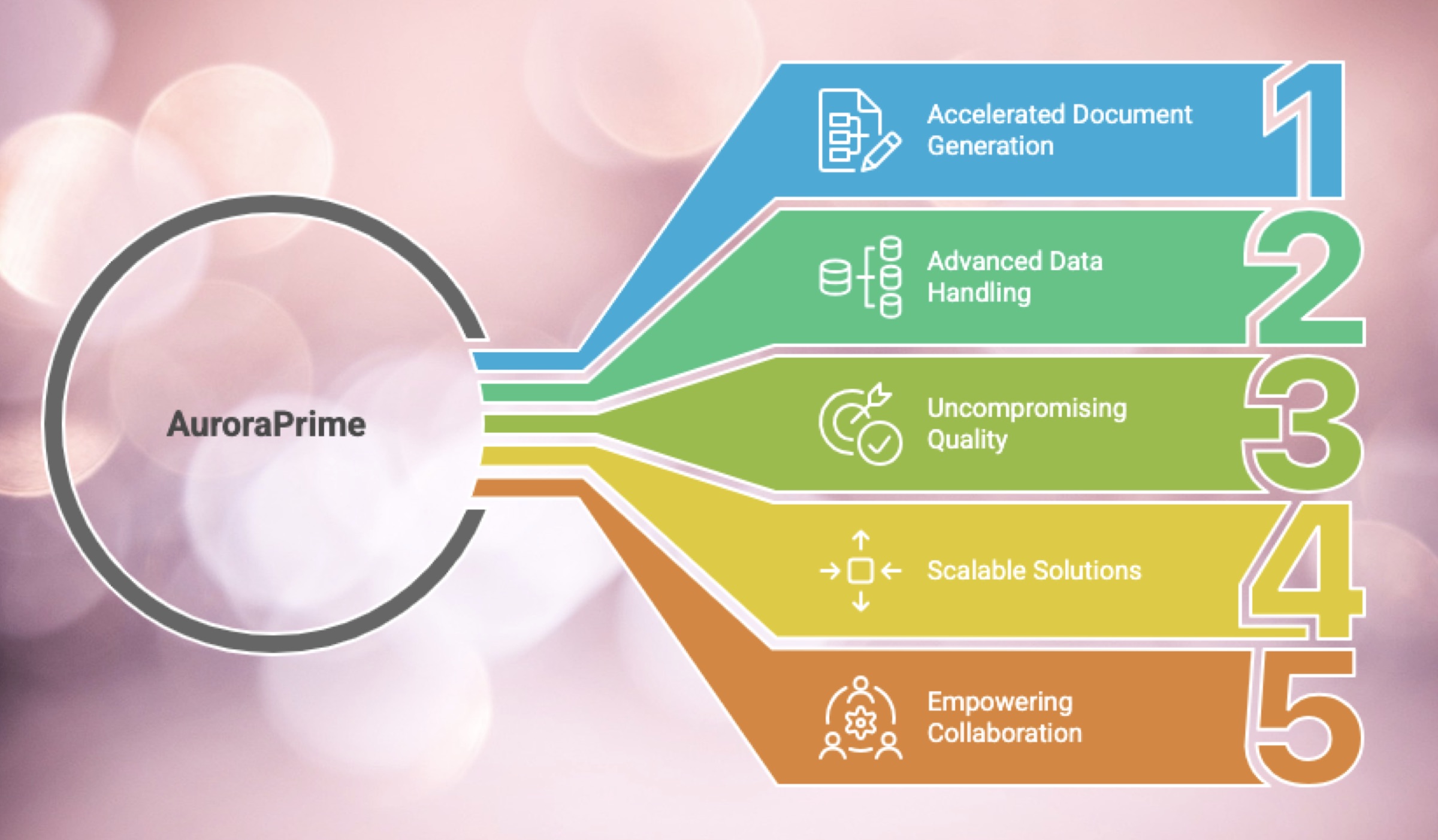
Accelerated Document Generation through AI Automation:
AuroraPrime empowers medical writers to auto-generate initial drafts of critical documents like Clinical Study Reports (CSRs), Protocols, and Patient Safety Narratives with remarkable speed. This automation leads to a 90% reduction in first draft time and a 50-70% overall time reduction for these documents, turning weeks into minutes.
It intelligently creates summaries of Tables, Figures, and Listings (TFLs) using AI, allowing users to provide custom prompts and examples to tailor the output. This process can be batch generated, updated, and validated asynchronously, freeing writers for other tasks.
The platform also supports batch generation of study protocol section content and batch TFL incorporation, significantly streamlining workflows.
Automated update triggers ensure documents stay current by monitoring linked source materials and automatically initiating revisions when changes occur upstream.
Advanced Data Handling & Seamless Integration:
A core strength of AuroraPrime RMA is its multimodal data ingestion capabilities, allowing it to seamlessly incorporate complex tabular data like TFLs from RTF and Excel files, and Schedules of Assessments (SOAs).
It features an improved merge table algorithm for complex source TFLs and supports manual identification of row and column headers for nuanced data structures, ensuring accurate processing.
AuroraPrime integrates effortlessly with existing enterprise systems such as Microsoft Word, Veeva Vault/RIM, Electronic Data Capture (EDC) platforms, and Pharmacovigilance (PV) systems. It also supports standard datasets like SDTM and ADaM.
Uncompromising Quality, Accuracy, and Compliance:
AuroraPrime features built-in GenAI QC (Quality Control) that benchmarks generated content against "golden" standards and performs automated integrity and consistency checks.
The "Validate TFL Summaries" feature compares generated summary text against corresponding TFL data, highlighting discrepancies and offering auto-updates to ensure factual accuracy.
The platform emphasizes Human-in-the-Loop (HITL) AI interaction, ensuring medical writers remain in control from content generation to final approval. User feedback on AI-generated content is actively captured to continuously improve AI performance.
AlphaLife Sciences designs AuroraPrime to adhere to rigorous global regulatory standards, including FDA 21 CFR Part 11, HIPAA, GDPR, ISO 9001, ISO 27001, and AICPA SOC 2 Type II. It can even generate and perform quality control of GxP reports automatically.
Scalable, Flexible, and Customizable Solutions:
Built on a modular architecture and low-code development framework (Compose™ Low-Code Framework and AI & LLM Workbench), AuroraPrime allows for ultimate flexibility and configurability. This enables rapid development and adaptation for new document types and therapeutic areas.
Template Admins can easily create and configure AI-enabled document templates from existing Word documents, define content generation rules, and include document tags for therapeutic areas, ensuring highly tailored and reusable content.
The platform supports a comprehensive and evolving range of clinical and regulatory documents, including CSRs, Protocols, Patient Safety Narratives, Development Safety Update Reports (DSURs), Periodic Safety Update Reports (PSURs), Clinical Overviews (Module 2.5), Summaries of Clinical Efficacy (Module 2.7.3), and Summaries of Clinical Safety (Module 2.7.4), among others.
Empowering Teams and Fostering Collaboration:
By automating repetitive and time-consuming tasks, AuroraPrime frees up medical writers and R&D leaders to focus on higher-value activities such as strategic tasks, scientific quality, and core innovation.
The platform offers native Microsoft Word 365 integration, providing a familiar and seamless environment for writers.
It streamlines teamwork with built-in collaboration tools and version tracking, enabling efficient review and refinement of drafts while maintaining alignment among stakeholders.
Partnering for Proven Success
AlphaLife Sciences isn't just envisioning the future; we're actively building and deploying it. AuroraPrime is a platform proven in global pharmaceutical deployments, trusted by five out of the top 10 global pharmaceutical companies and leading Contract Research Organizations (CROs).
Our clients are experiencing tangible benefits, with one top-10 global pharmaceutical company confirming a 30% time-saving in their Medical Writing Team through a pilot using two full historical CSRs. Another top-3 global CRO highlighted significant efficiency gains, particularly in content reuse, streamlining workflows for CSRs, Statistical Analysis Plans (SAPs), and study protocols.
The FDA recognizes the increasing adoption of AI in drug development and is committed to a risk-based regulatory framework that promotes innovation while ensuring drug safety and effectiveness. AuroraPrime's robust AI quality governance, detailed data traceability, and human-in-the-loop interaction align perfectly with this evolving regulatory landscape.
By automating complex workflows, ensuring data accuracy, and maintaining rigorous compliance, AlphaLife Sciences' AuroraPrime platform is empowering regulatory and medical writing teams to work with unparalleled speed, quality, and confidence. This not only accelerates regulatory approval and market access but fundamentally transforms the efficiency and impact of drug development for the entire life sciences industry.





