Data-Driven Decisions: Frost & Sullivan Confirms AI is the Strategic Imperative for Regulatory Excellence
Dec 11, 2025AI is rapidly becoming the backbone of modern regulatory and medical writing—and Frost & Sullivan’s new report makes it clear: the organizations moving now will define the future of regulatory excellence. 🚀With rising documentation demands and tighter timelines, leading pharma is turning to AI to boost quality, accelerate workflows, and free experts to focus on scientific judgment. 🔍✨ The industry shift is real, and it’s accelerating fast.
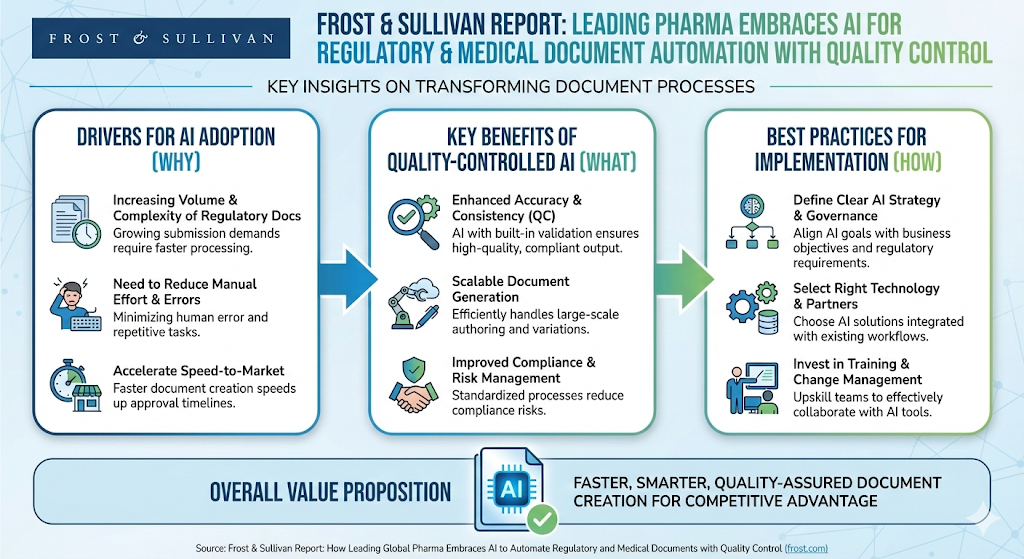
New report from Frost & Sullivan, released today, December 11, 2025, underscores a critical turning point for the pharmaceutical industry: the transition to AI-powered regulatory and medical documentation is no longer a strategic option but an essential capability. The new white paper, titled “How Leading Global Pharma Embraces AI to Automate Regulatory and Medical Documents with Quality Control,” synthesizes perspectives from regulatory agencies, biopharmaceutical leaders, and technology providers, confirming that the industry is undergoing rapid transformation to address mounting documentation demands and accelerate therapy delivery.
This comprehensive analysis validates the profound strategic shift underway among clinical operations, regulatory affairs, and medical writing leaders who are recognizing that automation and continuous improvement are essential to keep pace with rising scrutiny and operational demands.
The Transformation Imperative: Complexity Meets Cost Pressure
The report highlights that the need for transformation is rooted in the immense complexity and cost of drug development, where costs exceed $2.3B and timelines span 10–15 years. These factors mean that even small delays in documentation carry major impact. These pressures make regulatory writing a prime target for transformation, as manual processes have been outpaced by the volume and complexity required,. As Eunice Youhanna, Industry Advisor, Health & Life Sciences, Microsoft, noted, "AI-driven acceleration is no longer optional for pharma," given the significant financial impact of bringing a blockbuster drug to market even a month faster.
Leading with Vision: Executive Sponsorship and the Phased Roadmap
Successful AI adoption requires committed leadership to drive unified progress. According to the report, senior leaders must set the vision, focus on high-impact use cases, and ensure organizational readiness for change. AlphaLife Sciences Founder and CEO, Sharon Chen, emphasizes this strategic approach: "Align on the long-term goal, define the midterm value, and assess the short-term gain," noting that this clarity enables AI to become a truly transformative force when all executive stakeholders share the same understanding,. This methodology leads to a phased adoption framework, where organizations demonstrate short-term gains before moving toward long-term goals like end-to-end AI orchestration.
Quality Control and the Human Governor
A major theme explored by Frost & Sullivan is the necessary alignment between AI adoption and strict regulatory expectations. The report makes clear that AI is acceptable only when used within a transparent, well-controlled, human-governed framework. Regulatory expectations consistently center on accountability, traceability, and quality parity—meeting the same scientific and regulatory standards as human-authored documents.
AlphaLife Sciences anchors its thought leadership directly in this compliance mandate. Sharon Chen asserts that quality assurance must be "built specifically for regulatory content," which is where "real safety and trust are created". Furthermore, AI can serve as a quality safeguard: with embedded quality control, AI can "detect discrepancies that humans may overlook," strengthening both consistency and regulatory confidence across interconnected documents,. This focus ensures that quality, rather than speed alone, is the primary driver, aligning with the sentiment of former FDA Director John Jenkins, MD, who stresses that quality is the main concern, and reviewers "should not even know whether a document was or wasn’t generated with AI — the quality should be high and consistent".
The Strategic Roadmap: From Pilot to Production
Leading organizations are achieving consistent, measurable benefits by starting with "high volume, low risk" use cases, such as Clinical Study Reports (CSRs),. The report confirms that AI is exceptionally well suited for reporting and summarizing clinical results.
AlphaLife Sciences’ positioning validates the significant ROI achieved through this targeted strategy. When domain knowledge, structure, and quality control are built into the process, AI can reliably automate first-draft generation across the regulatory document lifecycle. Sharon Chen notes that "30–50% end-to-end lifecycle time reduction is consistently achievable" when multiple document types are onboarded together, not just for a single document. This level of acceleration—which Eric Henze, Senior Health Industry Digital Strategist at Microsoft, confirms is being seen in real customers—transforms AI from a tactical efficiency tool into a strategic capability embedded within the broader R&D and regulatory operating model.
AuroraPrime RMA: Operationalizing the Next Benchmark
Frost & Sullivan highlights that the market shift is being enabled by specialized, regulatory-focused AI platforms rather than generic tools. Solutions developed by providers such as AlphaLife Sciences were frequently cited as examples of "production-grade" GenAI for regulatory and medical writing.
AuroraPrime RMA aligns with the strict vendor criteria outlined in the report. The platform follows a design pattern that is human-centered, operating directly within Microsoft Word and integrating with RIM systems like Veeva to preserve version control and traceability,. This approach ensures AI "embeds into regulated workflows rather than attempt[ing] to replace them". Eric Henze reinforced this trust, stating that AlphaLife Sciences is "not a proof-of-concept startup"—it is already "in production across small, mid-size, and large enterprises". AlphaLife Sciences’ technical leadership is further validated through its role as Microsoft’s exclusive strategic AI content authoring partner for life sciences, and its enterprise-grade platform is already adopted by more than half of the world’s top-20 pharmaceutical companies.
The Future is Orchestrated
The report concludes that the industry is moving from AI-assisted drafting to full workflow orchestration. The next phase involves Agentic AI mapping the full hierarchy of information, ensuring alignment and consistency across entire submission packages. Sharon Chen emphasizes this necessity: "Agentic AI will ultimately map the full hierarchy of information, giving us a connected content ecosystem that delivers unmatched consistency, quality, and efficiency". This capability will enable organizations to achieve faster, smarter submissions, improving traceability and freeing up experts for strategic work.
The business case for AI is now compelling, and the future of regulatory excellence "will belong to organizations that operationalize AI today—not tomorrow," according to Sharon Chen.
We encourage all clinical operations, regulatory affairs, and medical writing leaders to explore the strategic implications of this landmark report.
Download the full Frost & Sullivan white paper here: https://alphalifesci.com/download/download-the-frost-sullivan-white-paper-how-leading-global-pharma-embraces-ai-to-automate-regulatory-and-medical-documents-with-quality-control





