Trusted by the world’s best
Industry Leadership and Certificates for Compliance
AlphaLife Sciences’ automated medical content authoring platform transforms document creation
- AI Automation: Generates accurate medical documents swiftly.
- Data Management: Organizes data and templates for easy access.
- Seamless Integration: Connects with existing systems for consistency.
- Regulatory Compliance: Checks documents for accuracy and compliance.
- Customizable Modules: Adapts workflows and document types as needed.
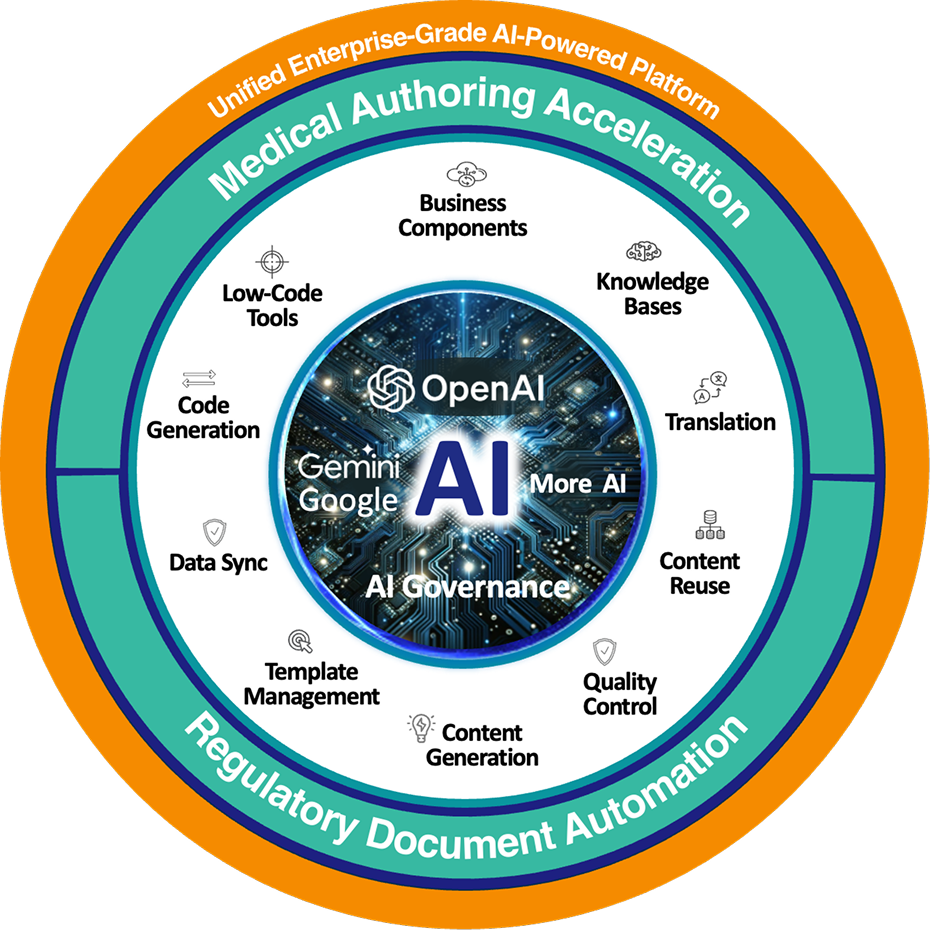
Expedite the Regulatory Document Development with the AuroraPrime Platform
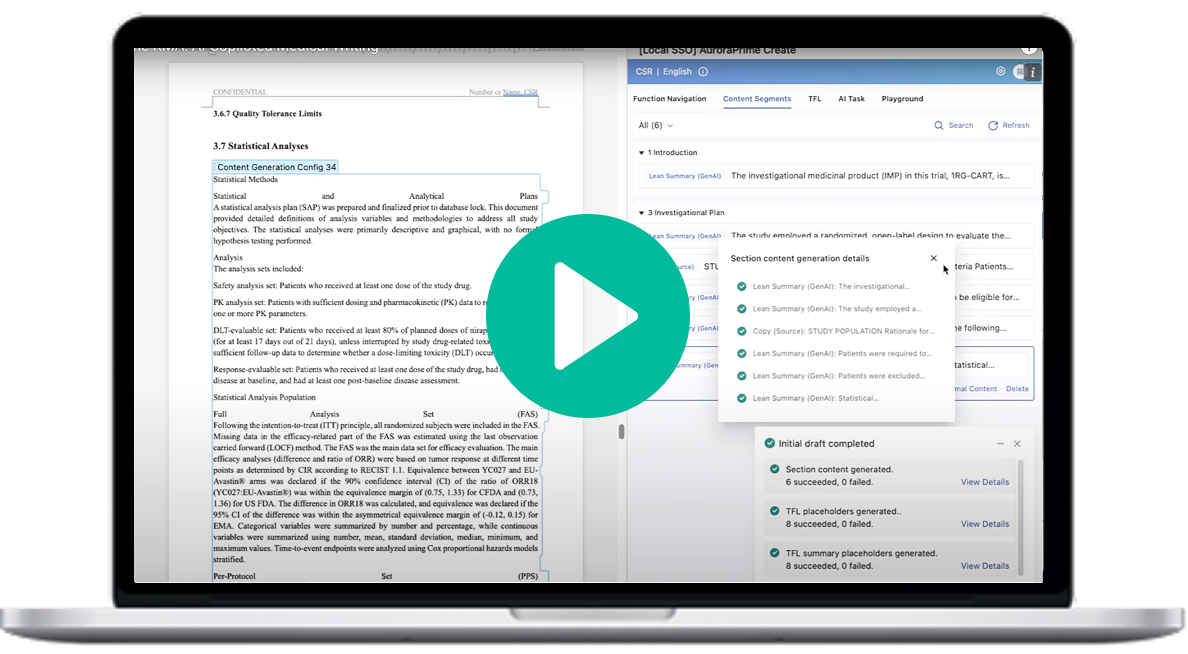
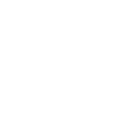
The AuroraPrime Platform, an advanced AI platform for medical content authoring, delivers agentic solutions that enhance the automated creation of regulatory documents across all phases of R&D. It supports pre-clinical, clinical, regulatory, and safety areas with a streamlined, intuitive interface for end-to-end content lifecycle management.
Enterprise System Integration
Real Results from Industry Leaders
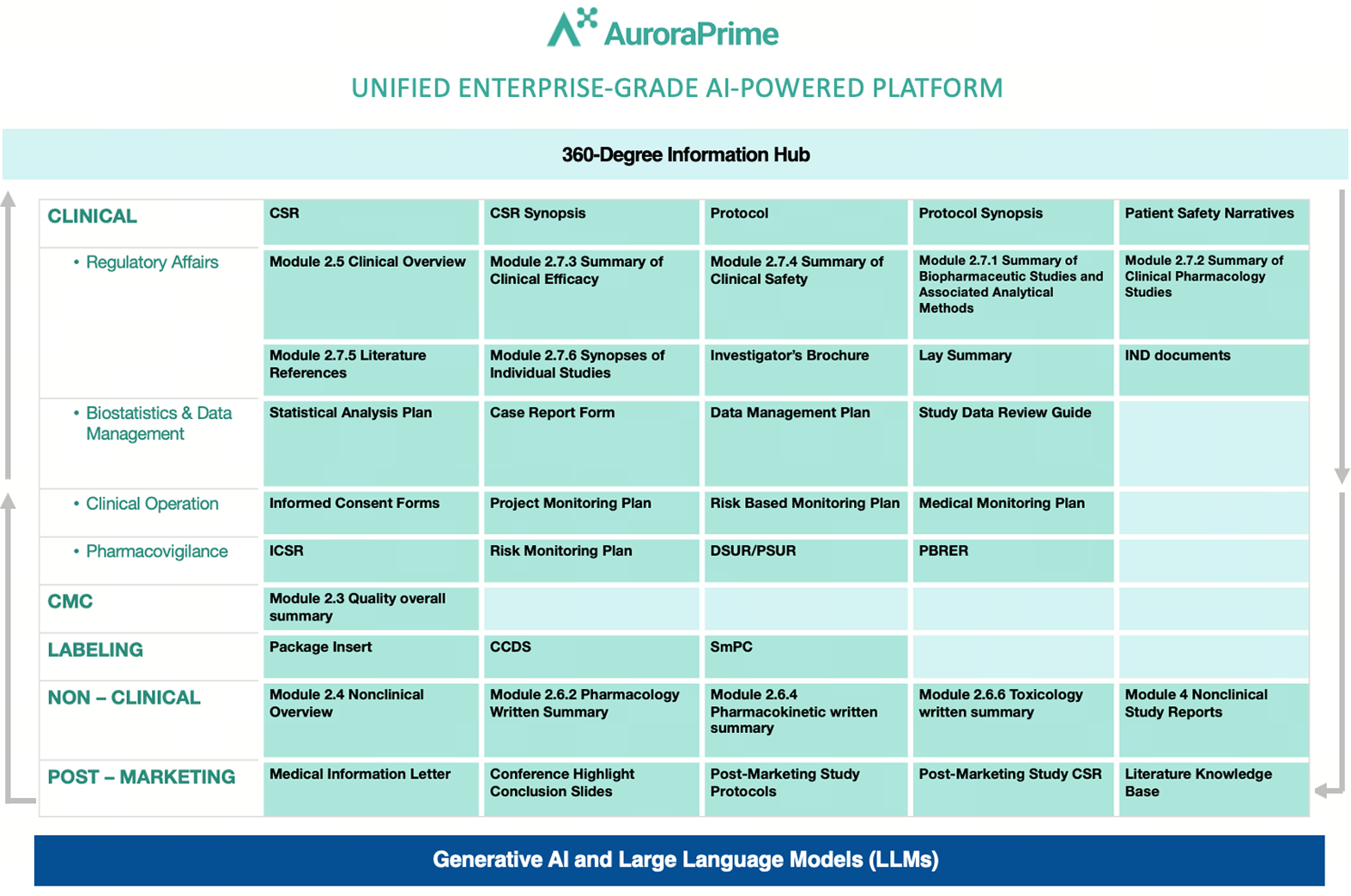
| Clinical Study Reports | Protocols | Safety Narratives | |
|---|---|---|---|
| Reduction in First Draft Time |
90% | 90% | 95% |
| Overall Time Savings |
50% | 50% | 70% |
Our AI-Powered Life Sciences Solutions
Empowering Life Sciences with AI-Driven Innovation:
A unified, AI-powered platform streamlining document
writing and generation, enhancing efficiency, and accelerating compliance for transformative outcomes.
AI-Powered
Document Authoring Enterprise Platform
- Enhance Quality and Efficiency: Streamline integration of data and documents, significantly improving output quality.
- Robust GenAI Architecture: Combine advanced AI technologies for precise, compliant document creation.
- Proven Time Savings: Reduce medical writing time by 30-90%, boosting productivity.
AI-Copiloted
Medical Writing Acceleration
- Template & Data Sync: Use enterprise templates and sync TFL data to streamline setup and drafting.
- Auto Content Generation: Quickly create TFL summaries, reducing manual work.
- Efficient Updates: Enable one-click batch updates, keeping documents current and compliant.
AI-Powered
Regulatory Document Automation
- Streamlined Regulatory Docs: Automate creation across pre-clinical, clinical, regulatory, and safety areas with GenAI.
- Built-in GenAI QC: Ensure precision by benchmarking against "golden" standards.
- Tailored Quality Metrics: Customize settings for task-specific evaluations, boosting accuracy and relevance.
Driving Innovation in Life Sciences with AI Excellence
Our platform combines deep expertise in life sciences and computing with cutting-edge AI integration to drive innovation. We ensure robust AI quality governance, offering a unified modular platform designed for scalability and low-code adaptability, empowering your enterprise to achieve transformative outcomes.
-

Life Sciences & Computing Domain Expertise
-
Life Sciences-Driven AI Excellence Solutions
-

Scalable and Flexible Enterprise Platform
Designed for Life Sciences Innovators & Pharmaceutical Leaders
Life Sciences Organizations Focused On R&D
AuroraPrime frees R&D leaders to focus on innovation by automating routine tasks, significantly boosting research efficiency and productivity.
Pharmaceutical Companies Seeking Regulatory Efficiency
Pharmaceutical companies benefit from our AI writing solutions, speeding up document creation for CSRs, Protocols, and Safety Narratives, ensuring compliance and quicker regulatory submissions.
Enterprises Aiming For Scalable AI-Driven Solutions
AuroraPrime supports extensive documentation needs with scalable solutions for various file types, customizable workflows, and integrations for rapid enterprise-wide deployment.
Why AlphaLife Sciences is Your Trusted Partner in AI Writing Solutions
Proven Success With Global Pharma Leaders
AuroraPrime frees R&D leaders to focus on innovation by automating routine tasks, significantly boosting research efficiency and productivity.
Advanced AI Integration Tailored for Life Sciences
AuroraPrime frees R&D leaders to focus on innovation by automating routine tasks, significantly boosting research efficiency and productivity.
Industry Certifications Ensuring Compliance
AlphaLife Sciences aligns with global regulatory standards, offering robust data protection and cost-effective compliance solutions tailored for the life sciences sector.
Flexible, Scalable Enterprise Solution
Our platform is uniquely scalable and flexible, integrating medical writing, data management, and analytics into one environment, enhanced by low-code adaptability for rapid deployment and customization.
Customer Success
“AlphaLife Sciences sets the industry standard. Your innovative solutions are transformative—enhancing efficiency and quality across our operations." —Top 5 Global Pharmaceutical Company
“By leveraging AlphaLife Sciences’ tools, we’ve achieved significant efficiency gains, particularly in content reuse. Starting with the CSR, we’ve streamlined workflows for the SAP and study protocols, saving time and reducing redundancy.” —Top 3 Global Leading CRO
Company News
Unlocking the Future of Life Sciences: A Recap from J.P. Morgan Healthcare Conference Week
From breakthrough dealmaking to hard conversations about scale, speed, and trust—J.P. Morgan Healthcare Conference Week once again made one thing clear: the future of life sciences will be built by those who can turn complexity into capability. 🧠🚀At AlphaLife Sciences, we saw a decisive shift from AI as promise to AI as infrastructure—powering regulatory intelligence, accelerating clinical development, and enabling medical and safety teams to operate with greater precision and confidence. This is not theoretical innovation; it is real-world transformation, already reshaping how decisions get made across the product lifecycle. 🔬📊Our recap captures the signals that matter most for regulatory, medical, and clinical leaders—and what they mean for organizations preparing for what’s next.
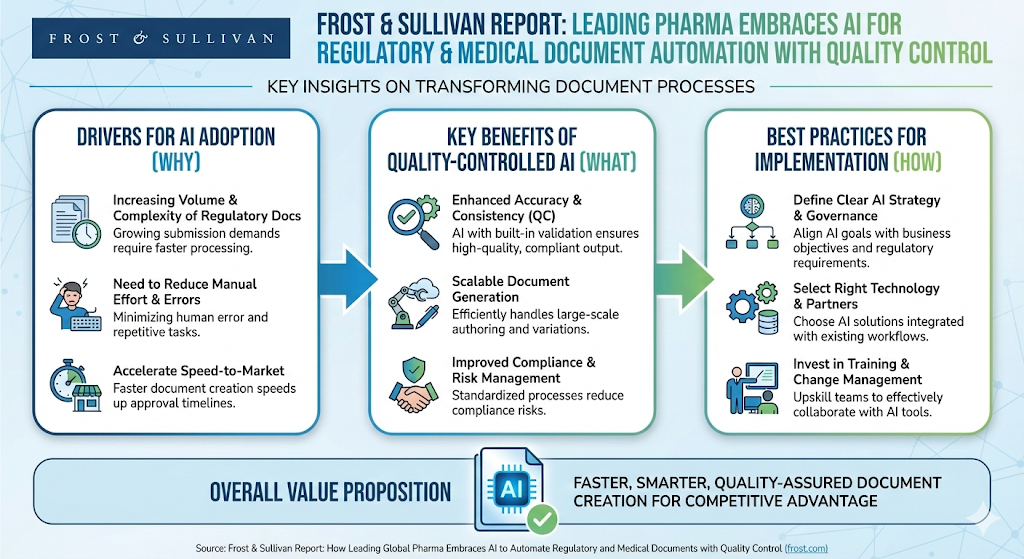
Jan 16, 2026Data-Driven Decisions: Frost & Sullivan Confirms AI is the Strategic Imperative for Regulatory Excellence
AI is rapidly becoming the backbone of modern regulatory and medical writing—and Frost & Sullivan’s new report makes it clear: the organizations moving now will define the future of regulatory excellence. 🚀With rising documentation demands and tighter timelines, leading pharma is turning to AI to boost quality, accelerate workflows, and free experts to focus on scientific judgment. 🔍✨ The industry shift is real, and it’s accelerating fast.
Dec 11, 2025The Human Element in AI: Quality, Strategy, and Compliance at DIA Webinar 2025
AI is accelerating faster than ever, but the real breakthroughs in life sciences happen when human expertise and machine intelligence move in lockstep. At this year’s DIA webinar, we explored how a human-centered approach to AI quality, strategy, and compliance is becoming the defining factor that separates pilots from scalable impact. 🚀 From regulatory reviewers to medical writers to clinical operations teams, the takeaway was clear: the future belongs to organizations that blend rigorous governance with empowered frontline teams — all supported by AI that learns continuously and safely within a unified, compliant framework. 🌐💡
Dec 02, 2025Beyond the First Draft: Elevating Quality and Compliance with Intelligent AI Agents at EMWA 2025
Imagine taking medical writing beyond the first draft—where AI doesn’t just assist but actively elevates quality, efficiency, and compliance. 🚀 At EMWA 2025, AlphaLife Sciences showcased how intelligent AI agents are transforming the regulatory and medical writing landscape, enabling teams to focus on strategy and insight rather than repetitive tasks. From real-world applications to measurable impact, this is AI designed for the life sciences, by life sciences. 🌐💡
Nov 26, 2025AMWA 2025 Deep Dive, Part II: The Gold Standard—Why Industry Leaders Called AuroraPrime RMA the "Regulatory Grade" AI for Medical Writing
🚀 What happens when regulatory rigor meets next-generation AI? At AMWA 2025, leaders across medical writing and regulatory affairs had a clear answer — AuroraPrime RMA is setting the new gold standard for regulatory-grade intelligence.From tackling the complexity of submission-ready outputs to transforming how teams navigate ever-evolving health authority expectations, the momentum is undeniable. This is more than automation — it’s a shift toward smarter, safer, and more scalable scientific communication across clinical, medical, and regulatory domains.If you’re curious why industry experts are calling this a defining moment for AI in life sciences, this deep dive is a must-read.
Nov 26, 2025